Conditional FDA Approval: Novavax COVID-19 Vaccine Faces Usage Limits
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Conditional FDA Approval: Novavax COVID-19 Vaccine Faces Usage Limits
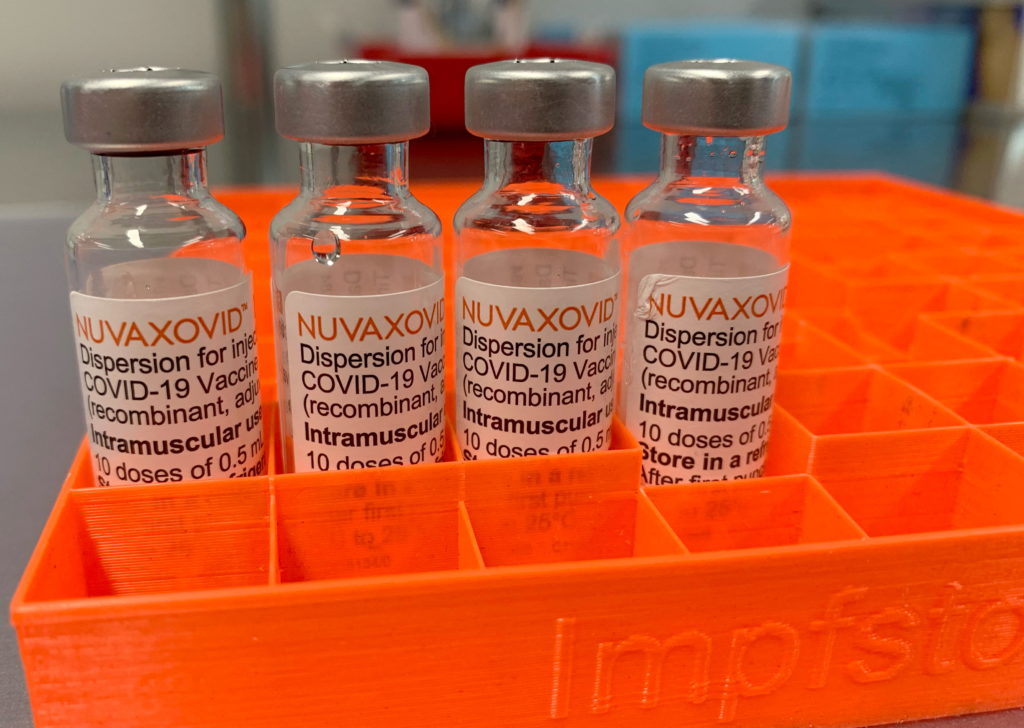
The Novavax COVID-19 vaccine, Nuvaxovid, has received conditional approval from the FDA, marking a significant development in the fight against the pandemic. However, this approval comes with limitations, raising questions about the vaccine's widespread adoption and impact. This article delves into the specifics of the FDA's decision, exploring the conditions attached to the approval and the implications for public health.
Limited Authorization: A Qualified Victory for Novavax
The FDA's approval is not a blanket endorsement. It's a conditional approval, meaning the authorization is contingent upon Novavax meeting ongoing requirements for safety and efficacy monitoring. This differs from a full approval, which signifies a more complete and rigorous review process. The conditional nature underscores the FDA's cautious approach, prioritizing public safety alongside the need for diverse vaccine options. This cautious approach is further emphasized by the limited age range for authorization.
Age Restrictions and Usage Guidelines
Currently, the FDA has authorized Nuvaxovid for individuals aged 18 and older. This restriction contrasts with other authorized COVID-19 vaccines, some of which have received approval for younger age groups. The limited age range could restrict the vaccine's potential reach and impact, particularly considering the ongoing need for vaccination among younger adults. The FDA's decision likely reflects ongoing data collection and analysis regarding the vaccine's safety and efficacy in younger populations. Further trials and data analysis will be crucial in determining whether the age restrictions can be lifted in the future.
What Makes Nuvaxovid Different?
Unlike mRNA vaccines like Pfizer-BioNTech and Moderna, Nuvaxovid uses a more traditional protein subunit technology. This technology involves using harmless pieces of the virus to trigger an immune response. This approach may appeal to some individuals hesitant about mRNA vaccines, offering an alternative option within the COVID-19 vaccination landscape. However, the effectiveness of this approach in the face of emerging variants remains a key area of ongoing research and monitoring.
Potential Impact on Vaccination Rates
The introduction of a new vaccine platform could potentially increase overall vaccination rates. Some individuals may prefer a protein-based vaccine due to perceived lower risk profiles compared to mRNA vaccines. However, the limited age range and conditional approval may limit its immediate impact. Public health initiatives will be crucial in educating the public about the vaccine's benefits and addressing any concerns or hesitancy. Further research is needed to fully understand its long-term efficacy and ability to combat evolving viral strains.
Looking Ahead: The Path to Full Approval
Novavax will need to meet stringent requirements to secure full FDA approval. This will likely involve ongoing surveillance of adverse events, long-term efficacy data, and further clinical trials. The timeline for full approval remains uncertain but will significantly depend on the success of these ongoing post-market surveillance efforts.
Conclusion: A Step Forward, But Challenges Remain
The conditional FDA approval of the Novavax COVID-19 vaccine represents a significant step forward in providing diverse vaccine options. However, the limited age range and the conditional nature of the approval highlight the need for continued monitoring and research. The long-term impact of Nuvaxovid on vaccination rates and the global fight against COVID-19 will depend heavily on addressing these limitations and ensuring widespread public awareness and acceptance. The future efficacy against emerging variants is also a key area of ongoing concern and research.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval: Novavax COVID-19 Vaccine Faces Usage Limits. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Misbehaving Tourists Beware Bali Implements New Guidelines
May 20, 2025
Misbehaving Tourists Beware Bali Implements New Guidelines
May 20, 2025 -
 Record Investments Over 5 B Poured Into Bitcoin Etfs Whats Driving The Rally
May 20, 2025
Record Investments Over 5 B Poured Into Bitcoin Etfs Whats Driving The Rally
May 20, 2025 -
 Freaky Friday Stars Jamie Lee Curtis Spills On Her Bond With Lindsay Lohan
May 20, 2025
Freaky Friday Stars Jamie Lee Curtis Spills On Her Bond With Lindsay Lohan
May 20, 2025 -
 Jamie Lee Curtis Discusses Her Close Bond With Lindsay Lohan
May 20, 2025
Jamie Lee Curtis Discusses Her Close Bond With Lindsay Lohan
May 20, 2025 -
 Mlb Betting Picks Best Walk Off Wagers For White Sox Vs Cubs Red Sox Vs Braves
May 20, 2025
Mlb Betting Picks Best Walk Off Wagers For White Sox Vs Cubs Red Sox Vs Braves
May 20, 2025
Latest Posts
-
 Cnn Reports Israeli Strikes Hit Only Remaining Hospital In North Gaza
May 20, 2025
Cnn Reports Israeli Strikes Hit Only Remaining Hospital In North Gaza
May 20, 2025 -
 Eu Uk Brexit Talks A Delicate Balance On The Brink Of Collapse
May 20, 2025
Eu Uk Brexit Talks A Delicate Balance On The Brink Of Collapse
May 20, 2025 -
 The Real Story Behind Jamie Lee Curtis And Lindsay Lohans Bond
May 20, 2025
The Real Story Behind Jamie Lee Curtis And Lindsay Lohans Bond
May 20, 2025 -
 Peaky Blinders Creator Reveals Plans For A New Series And A Significant Shift
May 20, 2025
Peaky Blinders Creator Reveals Plans For A New Series And A Significant Shift
May 20, 2025 -
 Bold Bets Drive 5 B Influx Into Bitcoin Etfs Market Analysis And Predictions
May 20, 2025
Bold Bets Drive 5 B Influx Into Bitcoin Etfs Market Analysis And Predictions
May 20, 2025
