Conditional FDA Approval: Novavax COVID-19 Vaccine's Limited Rollout
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
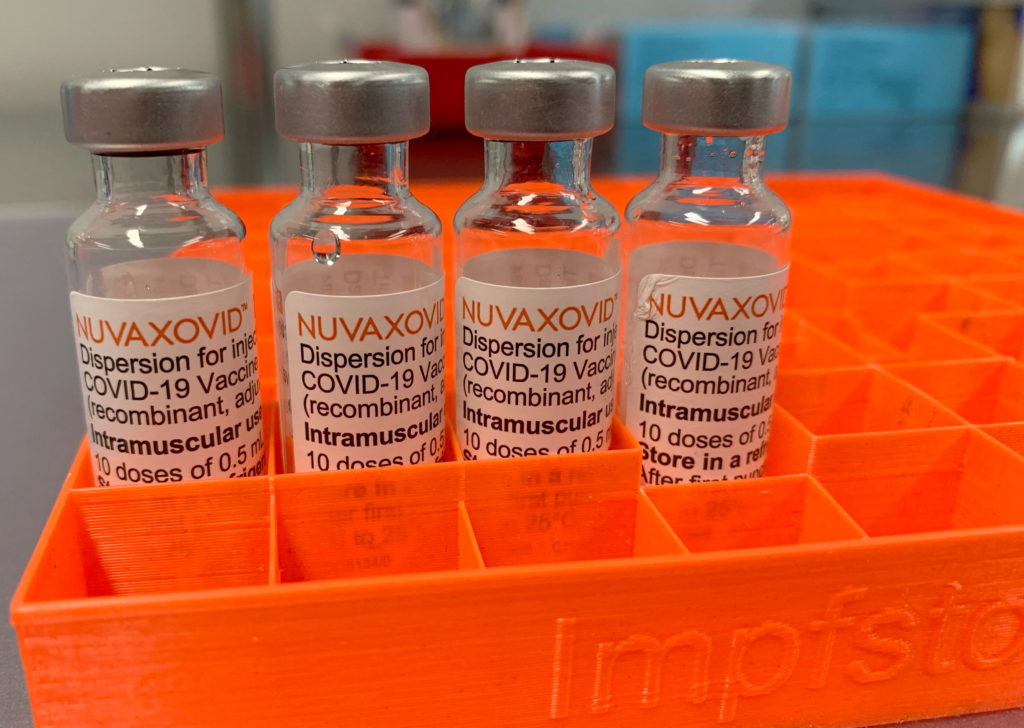
Conditional FDA Approval: Novavax COVID-19 Vaccine's Limited Rollout
The FDA's conditional approval of the Novavax COVID-19 vaccine, NVX-CoV2373, has sparked a wave of both excitement and cautious optimism. While hailed as a potential game-changer for vaccine hesitant individuals, its limited rollout raises questions about accessibility and overall impact on the pandemic's trajectory. This article delves into the specifics of the approval, the reasons behind the limited distribution, and what it means for the future of COVID-19 vaccination efforts.
A Traditional Approach in a Changing Landscape
Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, Novavax's vaccine utilizes a more traditional protein subunit technology. This approach, involving purified viral proteins, has been used for decades in vaccines against diseases like Hepatitis B and influenza. This familiarity might appeal to individuals wary of the newer mRNA technology, offering a potentially safer alternative in their eyes. However, this traditional approach also presents challenges in production and distribution, contributing to the vaccine's delayed arrival and current limited availability.
Why the Limited Rollout?
Several factors contribute to the Novavax vaccine's restrained launch:
-
Delayed Production: The manufacturing process, though familiar, proved more complex and time-consuming than initially anticipated, leading to significant production delays. This has directly impacted the number of doses available for immediate distribution.
-
Lower Demand: With high vaccination rates already achieved through other vaccines, the demand for a new option, even one using a different technology, has been less than initially projected. This lower demand further impacts the incentive for widespread distribution.
-
Efficacy Concerns: While effective, the Novavax vaccine's efficacy rates, while sufficient for approval, are slightly lower than those observed with the mRNA vaccines. This difference, though statistically significant, might contribute to a perceived lower priority in the current vaccination landscape.
-
Competition in the Market: The existing vaccines have established robust distribution networks and public confidence. Competing with these already-established vaccines for market share poses a significant challenge for Novavax.
Who Should Consider the Novavax Vaccine?
The Novavax vaccine might be a suitable option for individuals who:
- Are hesitant towards mRNA vaccines: The protein subunit technology might alleviate concerns regarding the novel mRNA approach.
- Have specific medical contraindications to other vaccines: Individual circumstances may make the Novavax vaccine a more appropriate choice.
It's crucial to consult with a healthcare provider to determine the most suitable vaccine based on individual medical history and risk factors.
The Future of Novavax's COVID-19 Vaccine
The future of the Novavax vaccine remains uncertain. While the conditional approval is a significant step, its success hinges on increased production, effective distribution strategies, and addressing public perception. The vaccine's role in combating future COVID-19 variants and its potential inclusion in booster campaigns will also play a crucial role in its long-term significance.
The FDA's approval underscores the importance of continued innovation in vaccine development. However, the challenges faced by Novavax highlight the complexities of transitioning from laboratory research to widespread deployment, especially in a rapidly evolving public health crisis. Ongoing monitoring of the vaccine's efficacy and safety, coupled with transparent communication, will be vital for building trust and maximizing its potential impact.
Call to Action: Consult your doctor to learn more about COVID-19 vaccines and determine which option is best for you. Stay informed about updates on vaccine availability and efficacy by following reputable sources like the CDC and WHO.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval: Novavax COVID-19 Vaccine's Limited Rollout. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Putin Demonstrates Trumps Waning International Leverage
May 20, 2025
Putin Demonstrates Trumps Waning International Leverage
May 20, 2025 -
 Missing Woman Found Alive Her Incredible Story Of Survival
May 20, 2025
Missing Woman Found Alive Her Incredible Story Of Survival
May 20, 2025 -
 Final Toluca Vs America Liga Mx 2025 Todo Sobre La Fecha Y Horario
May 20, 2025
Final Toluca Vs America Liga Mx 2025 Todo Sobre La Fecha Y Horario
May 20, 2025 -
 Brexit Endgame Eu Negotiations Reach Critical Point Amidst Betrayal Claims
May 20, 2025
Brexit Endgame Eu Negotiations Reach Critical Point Amidst Betrayal Claims
May 20, 2025 -
 2027 The Likely Uk Launch Date For Driverless Cars Uber Disagrees
May 20, 2025
2027 The Likely Uk Launch Date For Driverless Cars Uber Disagrees
May 20, 2025
Latest Posts
-
 Shifting Sands How The Trump Putin Call Impacts Ukraines Future
May 21, 2025
Shifting Sands How The Trump Putin Call Impacts Ukraines Future
May 21, 2025 -
 Analysis Trump Putin And The Changing Landscape Of Ukraine Peace Efforts
May 21, 2025
Analysis Trump Putin And The Changing Landscape Of Ukraine Peace Efforts
May 21, 2025 -
 Ingenious Or Inept Cats Prison Drug Run Ends In Capture Costa Rica
May 21, 2025
Ingenious Or Inept Cats Prison Drug Run Ends In Capture Costa Rica
May 21, 2025 -
 Tim Dillon On Cnn A Comedians Perspective On Political Interviews
May 21, 2025
Tim Dillon On Cnn A Comedians Perspective On Political Interviews
May 21, 2025 -
 Fbi Investigation Into New York Attorney General What We Know
May 21, 2025
Fbi Investigation Into New York Attorney General What We Know
May 21, 2025
