Conditional FDA Approval: Novavax COVID-19 Vaccine's Restricted Use
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Conditional FDA Approval: Novavax COVID-19 Vaccine's Restricted Use
The FDA's conditional approval of the Novavax COVID-19 vaccine marks a significant, albeit limited, step in the ongoing battle against the pandemic. While heralded by some as offering a different approach for vaccine-hesitant individuals, its restricted use raises important questions about accessibility and overall impact. This article delves into the specifics of the approval, exploring the reasons behind the restrictions and examining the potential implications for public health.
Understanding the Conditional Approval:
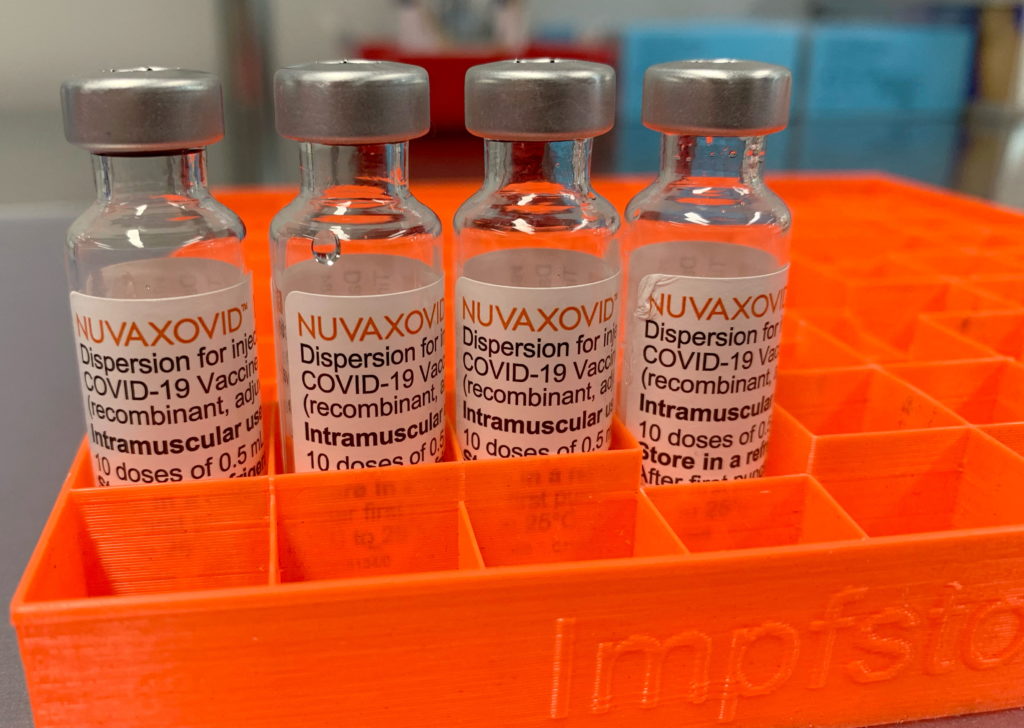
The U.S. Food and Drug Administration (FDA) granted conditional approval to Novavax's COVID-19 vaccine, Nuvaxovid, for individuals 18 years of age and older. This conditional approval, unlike a full licensure, means the FDA will continue to monitor the vaccine's safety and effectiveness post-market. This is standard practice for novel vaccines, especially during public health emergencies. The conditional approval hinges on the submission of further data by Novavax, demonstrating long-term safety and efficacy.
Why the Restrictions?
Several factors contribute to the restricted use of the Novavax vaccine:
- Limited Data: While clinical trials showed promising results, the FDA requires more extensive long-term data on the vaccine's safety and efficacy before granting full approval. This is crucial for understanding potential long-term side effects and ensuring its continued effectiveness against emerging variants.
- Manufacturing Capacity: The initial manufacturing capacity for the Novavax vaccine is relatively limited compared to other widely available COVID-19 vaccines like Pfizer-BioNTech and Moderna. This constraint directly impacts the vaccine's accessibility.
- Market Saturation: The demand for COVID-19 vaccines has decreased significantly since the initial rollout. With other vaccines readily available, the need for another vaccine may seem less urgent, impacting the overall rollout strategy.
Novavax's Unique Technology:
Unlike mRNA vaccines (Pfizer-BioNTech and Moderna), Novavax utilizes a protein subunit technology. This involves using parts of the virus (the spike protein) to trigger an immune response. This technology has been used in other vaccines for years and might appeal to individuals hesitant about mRNA vaccines due to their novel technology. This aspect is crucial for understanding the potential role of Novavax in addressing vaccine hesitancy. However, it's important to note that efficacy against current variants remains a key area of ongoing research and evaluation.
The Road Ahead:
The conditional approval of the Novavax vaccine presents both opportunities and challenges. Its unique technology offers a potential alternative for those hesitant towards mRNA vaccines. However, the limited availability and ongoing need for further data highlight the complexities of vaccine deployment during a pandemic. The FDA's continued monitoring is essential for ensuring the long-term safety and efficacy of the vaccine and informing future public health strategies. Further research into its effectiveness against emerging variants is also crucial.
Call to Action:
Consult your physician to discuss which COVID-19 vaccine is right for you. Staying informed about vaccine safety and efficacy is vital for making informed healthcare decisions. For more information on COVID-19 vaccines, visit the .

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval: Novavax COVID-19 Vaccine's Restricted Use. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Red Carpet Etiquette Why Guests Continue To Defy The Dress Code And Protocol
May 20, 2025
Red Carpet Etiquette Why Guests Continue To Defy The Dress Code And Protocol
May 20, 2025 -
 Back To Back Murders Colombian Model And Mexican Influencer Deaths Spark Outrage Over Femicide
May 20, 2025
Back To Back Murders Colombian Model And Mexican Influencer Deaths Spark Outrage Over Femicide
May 20, 2025 -
 Dallas Fort Worth Tornado Watch Expiry Latest Weather Reports
May 20, 2025
Dallas Fort Worth Tornado Watch Expiry Latest Weather Reports
May 20, 2025 -
 Femicide A Deep Dive Into The Causes Of Increased Violence Against Women
May 20, 2025
Femicide A Deep Dive Into The Causes Of Increased Violence Against Women
May 20, 2025 -
 Netflixs Fall Of Favre Director On Balancing Legacy And Scandals
May 20, 2025
Netflixs Fall Of Favre Director On Balancing Legacy And Scandals
May 20, 2025
Latest Posts
-
 Beyond The Screen Jamie Lee Curtis Shares Her Heartfelt Feelings For Lindsay Lohan
May 20, 2025
Beyond The Screen Jamie Lee Curtis Shares Her Heartfelt Feelings For Lindsay Lohan
May 20, 2025 -
 Cnn Reports Israeli Strikes Hit Only Remaining Hospital In North Gaza
May 20, 2025
Cnn Reports Israeli Strikes Hit Only Remaining Hospital In North Gaza
May 20, 2025 -
 Eu Uk Brexit Talks A Delicate Balance On The Brink Of Collapse
May 20, 2025
Eu Uk Brexit Talks A Delicate Balance On The Brink Of Collapse
May 20, 2025 -
 The Real Story Behind Jamie Lee Curtis And Lindsay Lohans Bond
May 20, 2025
The Real Story Behind Jamie Lee Curtis And Lindsay Lohans Bond
May 20, 2025 -
 Peaky Blinders Creator Reveals Plans For A New Series And A Significant Shift
May 20, 2025
Peaky Blinders Creator Reveals Plans For A New Series And A Significant Shift
May 20, 2025
