Conditional FDA Approval: Understanding The Novavax COVID-19 Vaccine Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Conditional FDA Approval: Understanding the Novavax COVID-19 Vaccine Restrictions
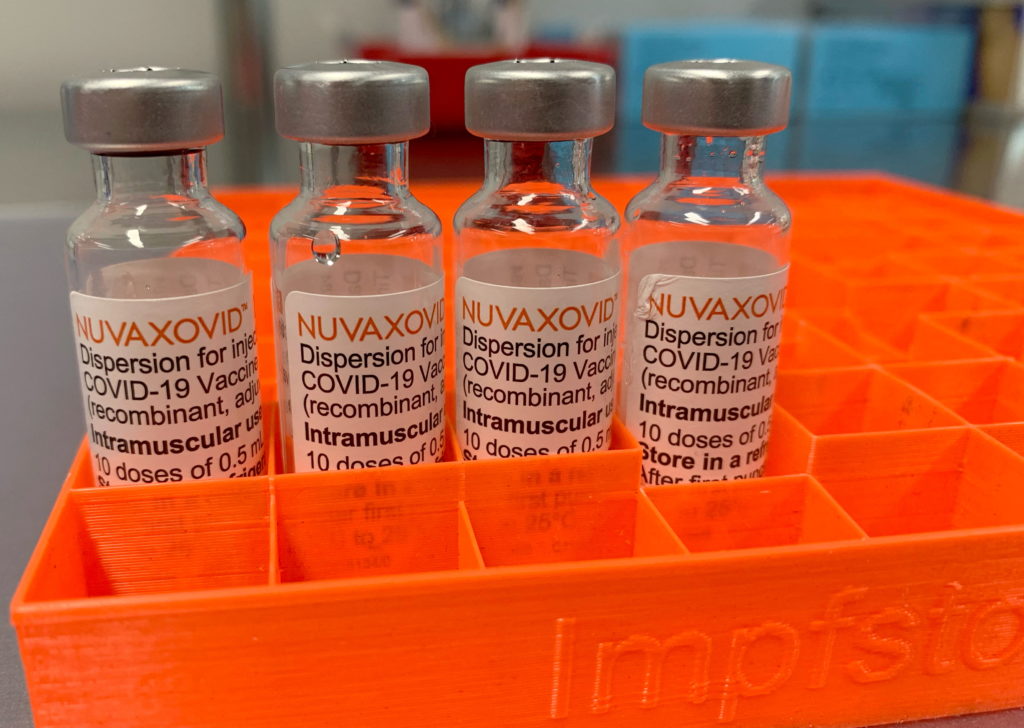
The Novavax COVID-19 vaccine, marketed as Nuvaxovid, finally received conditional FDA approval in July 2023, offering a different approach to COVID-19 vaccination. However, this approval wasn't without conditions. Understanding these restrictions is crucial for both healthcare providers and the public to make informed decisions about its use. This article delves into the specifics of the FDA's conditional approval and what it means for you.
What is Conditional FDA Approval?
Unlike a full FDA approval, which signifies extensive long-term safety and efficacy data, conditional approval allows the use of a vaccine during a public health emergency, based on more limited but still substantial data. This allows faster access to potentially life-saving treatments while further studies continue to gather comprehensive information. The FDA maintains rigorous oversight, and the approval can be revoked if subsequent data reveals safety concerns or insufficient efficacy.
Novavax Vaccine: A Different Technology
The Novavax vaccine stands apart from the mRNA vaccines (Pfizer-BioNTech and Moderna) and the viral vector vaccine (Johnson & Johnson) primarily due to its technology. It uses a protein subunit technology, employing a lab-created version of the spike protein found on the surface of the SARS-CoV-2 virus. This protein is then combined with an adjuvant to boost the immune response. This approach may appeal to individuals hesitant about mRNA or viral vector vaccines. [Link to CDC page on different vaccine technologies]
The FDA's Conditions for Approval:
The FDA's conditional approval for the Novavax vaccine came with several stipulations, including:
- Ongoing Safety Monitoring: Novavax, like all vaccine manufacturers, is required to continue monitoring the vaccine's safety profile through post-market surveillance. This involves tracking adverse events and analyzing long-term safety data.
- Further Clinical Trials: Additional clinical trials are mandated to further solidify the vaccine's efficacy and safety data, particularly regarding long-term effects and different populations.
- Manufacturing Requirements: Strict manufacturing standards must be maintained to ensure consistent quality and potency.
Who Should Consider the Novavax Vaccine?
The Novavax vaccine is authorized for individuals 18 years and older. While it presents a viable alternative, it's essential to consult with a healthcare professional to determine if it's the right choice for you. Factors to consider include:
- Pre-existing medical conditions: Individuals with certain medical conditions may need to discuss potential risks and benefits with their doctor.
- Previous COVID-19 infection: The need for vaccination after a previous infection is a subject of ongoing discussion and should be discussed with a physician.
- Vaccine hesitancy based on technology: The protein subunit technology of the Novavax vaccine might appeal to individuals hesitant about mRNA or viral vector vaccines.
Addressing Concerns and Misinformation:
Several misconceptions surround the Novavax vaccine. It's crucial to rely on credible sources of information, such as the FDA and CDC websites, to avoid misinformation. The FDA's conditional approval is not an indication of a lack of efficacy or safety; rather, it reflects the ongoing nature of scientific understanding and the need for continuous monitoring.
Conclusion:
The conditional FDA approval of the Novavax COVID-19 vaccine provides a valuable alternative for individuals seeking a different type of COVID-19 vaccination. However, understanding the associated conditions and consulting with a healthcare professional is paramount in making an informed decision. Staying informed through reputable sources like the CDC and FDA websites will help you navigate this evolving landscape of COVID-19 vaccination.
Call to Action: Talk to your doctor to determine which COVID-19 vaccine is right for you.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval: Understanding The Novavax COVID-19 Vaccine Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Official Statement President Biden Receives Prostate Cancer Diagnosis
May 21, 2025
Official Statement President Biden Receives Prostate Cancer Diagnosis
May 21, 2025 -
 Ellen De Generes Heartbreak Publicly Mourning An Irreplaceable Loss
May 21, 2025
Ellen De Generes Heartbreak Publicly Mourning An Irreplaceable Loss
May 21, 2025 -
 Weaning From Pacifiers And Thumb Sucking Age And Strategies For Parents
May 21, 2025
Weaning From Pacifiers And Thumb Sucking Age And Strategies For Parents
May 21, 2025 -
 Gaza Conflict And Brexit Two Major Global Headlines
May 21, 2025
Gaza Conflict And Brexit Two Major Global Headlines
May 21, 2025 -
 St Louis Tornado Aftermath How The Community Is Picking Up The Pieces
May 21, 2025
St Louis Tornado Aftermath How The Community Is Picking Up The Pieces
May 21, 2025
Latest Posts
-
 Police Investigate Church Break In Report Of Defecation
May 21, 2025
Police Investigate Church Break In Report Of Defecation
May 21, 2025 -
 Trump Doj And James A Complex Web Of Legal Battles
May 21, 2025
Trump Doj And James A Complex Web Of Legal Battles
May 21, 2025 -
 Diplomatic Tensions Rise As Allies Demand End To Israels Gaza Operation
May 21, 2025
Diplomatic Tensions Rise As Allies Demand End To Israels Gaza Operation
May 21, 2025 -
 New Orleans Jailbreak Fourth Inmate Captured As District Attorneys Staff Seek Safety
May 21, 2025
New Orleans Jailbreak Fourth Inmate Captured As District Attorneys Staff Seek Safety
May 21, 2025 -
 Police Teenagers Arrested For Urinating And Defecating In Santa Rosa Church
May 21, 2025
Police Teenagers Arrested For Urinating And Defecating In Santa Rosa Church
May 21, 2025
