Conditional FDA Approval: Understanding The Novavax COVID-19 Vaccine's Limitations
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Conditional FDA Approval: Understanding the Novavax COVID-19 Vaccine's Limitations
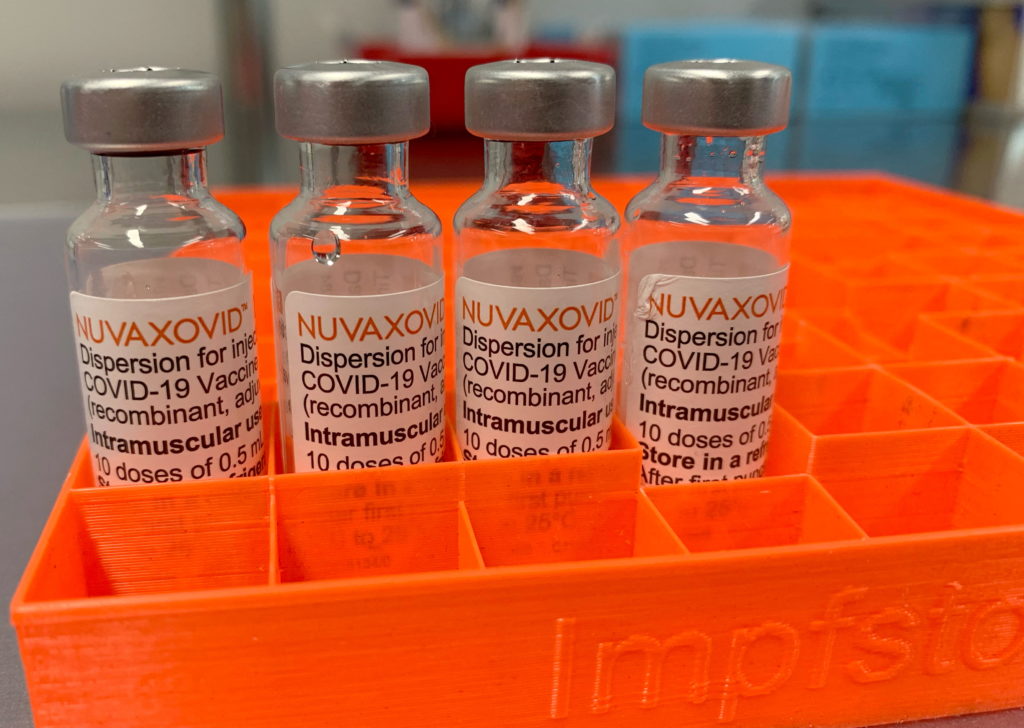
The Novavax COVID-19 vaccine, Nuvaxovid, finally received conditional FDA approval in July 2023, offering a different approach to COVID-19 immunization. However, its arrival wasn't without caveats. This article delves into the specifics of the FDA's conditional approval, highlighting the vaccine's limitations and what it means for individuals considering this option.
What is Conditional FDA Approval?
Unlike a full FDA approval, conditional approval allows for the use of a vaccine or medication based on a shorter dataset of clinical trial information. This accelerated pathway is typically used during public health emergencies, like the COVID-19 pandemic, to expedite access to potentially life-saving treatments. It's important to note that conditional approval isn't a sign of inferior quality; rather, it reflects a faster regulatory process with ongoing data collection and analysis post-market release. The FDA will continue to monitor the vaccine's safety and efficacy after conditional approval, potentially leading to full approval or withdrawal depending on the findings.
Novavax's Unique Approach: Protein Subunit Technology
Novavax's Nuvaxovid distinguishes itself from mRNA vaccines (like Pfizer-BioNTech and Moderna) and viral vector vaccines (like Johnson & Johnson's Janssen vaccine) by utilizing a protein subunit technology. This method involves using harmless pieces of the virus's protein to trigger an immune response. This approach is considered more traditional and may appeal to individuals hesitant about mRNA or viral vector technologies. However, this traditional approach also impacts its efficacy and limitations.
Limitations of the Novavax COVID-19 Vaccine:
-
Lower Efficacy: Clinical trials have demonstrated lower efficacy compared to mRNA vaccines, particularly against emerging variants. While effective in preventing severe illness, hospitalization, and death, its protection against milder infections might be less robust. [Link to relevant FDA data/study].
-
Logistical Challenges: The vaccine requires specific storage and handling conditions, potentially making distribution more complex than other COVID-19 vaccines.
-
Limited Data on Long-Term Effects: Due to the accelerated approval process, long-term data on the vaccine's effects is still limited. Ongoing monitoring is crucial to fully assess its long-term safety and efficacy profile.
-
Adverse Effects: Like all vaccines, Nuvaxovid can cause side effects. While generally mild (pain at the injection site, fatigue, headache), serious adverse events have been reported, though rarely. [Link to CDC or FDA adverse event reporting system].
Who Should Consider the Novavax Vaccine?
The Novavax vaccine might be a suitable option for individuals:
- Hesitant about mRNA or viral vector vaccines: Its protein subunit technology may alleviate concerns regarding these other vaccine platforms.
- Seeking a different vaccine option: Having multiple vaccine choices increases access and allows for personalized risk assessments.
Conclusion: Informed Decision-Making is Key
The conditional FDA approval of the Novavax COVID-19 vaccine provides an additional tool in the fight against the pandemic. However, understanding its limitations is critical for informed decision-making. Individuals should discuss their medical history and vaccine preferences with their healthcare provider to determine the most appropriate COVID-19 vaccine for their individual circumstances. Remember to consult reliable sources like the FDA and CDC for the most up-to-date information on vaccine safety and efficacy. Staying informed is vital in navigating this evolving public health landscape.
Call to Action: Talk to your doctor today to learn more about your COVID-19 vaccination options.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval: Understanding The Novavax COVID-19 Vaccine's Limitations. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Jon Jones Retirement Rumors Intensify Aspinall Negotiations And The I M Done Statement
May 20, 2025
Jon Jones Retirement Rumors Intensify Aspinall Negotiations And The I M Done Statement
May 20, 2025 -
 Is Your Child Ready To Give Up Their Pacifier Or Thumb
May 20, 2025
Is Your Child Ready To Give Up Their Pacifier Or Thumb
May 20, 2025 -
 Fecha Y Hora De La Final Liga Mx 2025 Toluca Vs America
May 20, 2025
Fecha Y Hora De La Final Liga Mx 2025 Toluca Vs America
May 20, 2025 -
 President Biden Receives Prostate Cancer Diagnosis Impact On Presidency And Treatment Plan
May 20, 2025
President Biden Receives Prostate Cancer Diagnosis Impact On Presidency And Treatment Plan
May 20, 2025 -
 Buy Now Pay Later Understanding The Changes In Consumer Protection
May 20, 2025
Buy Now Pay Later Understanding The Changes In Consumer Protection
May 20, 2025
Latest Posts
-
 Urgent Action Needed Colombian Models Murder Follows Mexican Influencers Death Heightening Femicide Concerns
May 20, 2025
Urgent Action Needed Colombian Models Murder Follows Mexican Influencers Death Heightening Femicide Concerns
May 20, 2025 -
 Lufthansa Flight Operated Without Pilot For 10 Minutes A Safety Investigation Report
May 20, 2025
Lufthansa Flight Operated Without Pilot For 10 Minutes A Safety Investigation Report
May 20, 2025 -
 Jon Jones Retirement I M Done Comment Ignites Ufc Debate
May 20, 2025
Jon Jones Retirement I M Done Comment Ignites Ufc Debate
May 20, 2025 -
 Exclusive Interview The Making Of Netflixs Fall Of Favre And The Favre Legacy
May 20, 2025
Exclusive Interview The Making Of Netflixs Fall Of Favre And The Favre Legacy
May 20, 2025 -
 Record Bitcoin Etf Investments Strategic Bets Fueling Market Growth
May 20, 2025
Record Bitcoin Etf Investments Strategic Bets Fueling Market Growth
May 20, 2025
