FDA Approval For Novavax COVID-19 Vaccine Comes With Unusual Conditions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
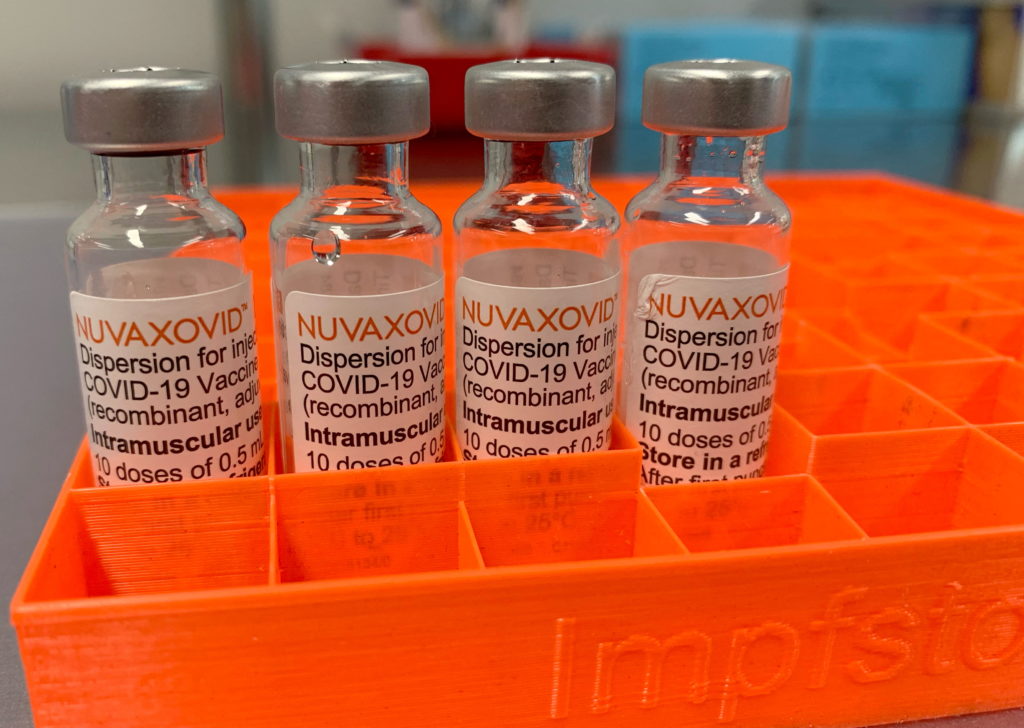
FDA Approval for Novavax COVID-19 Vaccine Comes with Unusual Conditions
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, has been met with a mixture of relief and surprise. While hailed as a victory for vaccine diversity and a potential boon for vaccine hesitant populations, the approval comes with several unusual conditions that have raised eyebrows within the scientific community and public health circles. This isn't your typical vaccine green light.
What Makes This Approval Different?
Unlike the rapid emergency use authorizations (EUAs) granted to Pfizer-BioNTech and Moderna vaccines, the Novavax vaccine's approval process was significantly longer and more rigorous. This lengthy review, however, wasn't solely due to scientific uncertainty. The FDA's decision to grant full approval, rather than sticking with an EUA, included several stipulations that significantly impact the vaccine's rollout and potential market reach.
The Unusual Conditions:
-
Limited Dosage: The FDA approval is specifically for a two-dose primary series, leaving the booster dose currently under review. This means the vaccine's overall effectiveness against evolving variants, particularly those like Omicron and its subvariants, remains somewhat uncertain until further data on booster efficacy becomes available. This limited authorization contrasts with the already established booster strategies for other authorized vaccines.
-
Manufacturing Constraints: Novavax has faced significant manufacturing hurdles, delaying the vaccine's widespread availability. The FDA's approval likely hinges on the company's ability to consistently produce the vaccine to meet demand. Any further production setbacks could hinder the vaccine's impact on public health efforts.
-
Stringent Monitoring: The FDA has implemented stricter post-market surveillance requirements for Nuvaxovid than for other approved COVID-19 vaccines. This enhanced monitoring will focus on identifying and addressing any unforeseen side effects or safety concerns that might emerge after widespread use. This heightened scrutiny underscores the FDA's cautious approach to this particular vaccine.
-
Target Population: Although approved for individuals 18 years and older, the vaccine's effectiveness in specific subpopulations, including the immunocompromised, may require further study before widespread recommendation. This necessitates a more targeted approach to distribution and public health messaging.
Why the Unusual Cautious Approach?
Several factors likely contributed to the FDA's cautious approach:
- Novel Technology: Novavax uses a protein subunit technology, different from the mRNA technology used by Pfizer-BioNTech and Moderna. While this technology has a long history of safety, its application to a novel virus like SARS-CoV-2 required a more thorough evaluation.
- Manufacturing Challenges: As mentioned, the manufacturing process has been complex and presented challenges, leading to delays and raising concerns about long-term supply.
- Public Perception: Gaining public trust after initial delays is crucial. A thorough review and careful rollout can help build confidence in the vaccine.
Looking Ahead:
The FDA's approval of the Novavax COVID-19 vaccine represents a significant step, yet the unusual conditions attached highlight the complexities of vaccine development and approval. The vaccine's long-term impact will depend heavily on addressing manufacturing challenges, conducting further research into booster efficacy and broader populations, and successfully navigating public perceptions. Its success will ultimately depend on the successful implementation of the FDA’s carefully crafted strategy.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein subunit vaccine, vaccine hesitancy, vaccine rollout, manufacturing challenges, post-market surveillance, vaccine safety, mRNA vaccines, COVID-19 booster
Related Articles: (These would link to other relevant articles on your site) [Link to article on COVID-19 vaccine efficacy] [Link to article on vaccine hesitancy] [Link to article on COVID-19 booster shots]

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine Comes With Unusual Conditions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Autonomous Vehicles In The Uk Ubers Early Adoption And The Regulatory Landscape
May 20, 2025
Autonomous Vehicles In The Uk Ubers Early Adoption And The Regulatory Landscape
May 20, 2025 -
 From Olympic Gold To Broken A Swimmers Story Of Abuse And Recovery
May 20, 2025
From Olympic Gold To Broken A Swimmers Story Of Abuse And Recovery
May 20, 2025 -
 Investigation Uncovers 10 Minute Pilotless Lufthansa Flight After Co Pilots Medical Emergency
May 20, 2025
Investigation Uncovers 10 Minute Pilotless Lufthansa Flight After Co Pilots Medical Emergency
May 20, 2025 -
 Juego De Voces 2025 En Vivo El Esperado Dueto De Yahir Y Victor Garcia
May 20, 2025
Juego De Voces 2025 En Vivo El Esperado Dueto De Yahir Y Victor Garcia
May 20, 2025 -
 How One Us Factory Highlights The Failures Of Trumps Trade Strategy
May 20, 2025
How One Us Factory Highlights The Failures Of Trumps Trade Strategy
May 20, 2025
Latest Posts
-
 President Bidens Prostate Cancer Diagnosis A Detailed Report
May 21, 2025
President Bidens Prostate Cancer Diagnosis A Detailed Report
May 21, 2025 -
 Lufthansa Co Pilots Fainting Flight Continues Pilotless For 10 Minutes
May 21, 2025
Lufthansa Co Pilots Fainting Flight Continues Pilotless For 10 Minutes
May 21, 2025 -
 Violence Against Women Colombian Model And Mexican Influencer Killed Sparking Global Condemnation
May 21, 2025
Violence Against Women Colombian Model And Mexican Influencer Killed Sparking Global Condemnation
May 21, 2025 -
 Ufc News Jon Jones Hints At Retirement Amidst Aspinall Contract Delay
May 21, 2025
Ufc News Jon Jones Hints At Retirement Amidst Aspinall Contract Delay
May 21, 2025 -
 Latest Updates President Bidens Health And Prostate Cancer Treatment
May 21, 2025
Latest Updates President Bidens Health And Prostate Cancer Treatment
May 21, 2025
