FDA Approval For Novavax COVID-19 Vaccine Comes With Unusual Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Approval for Novavax COVID-19 Vaccine Comes with Unusual Restrictions
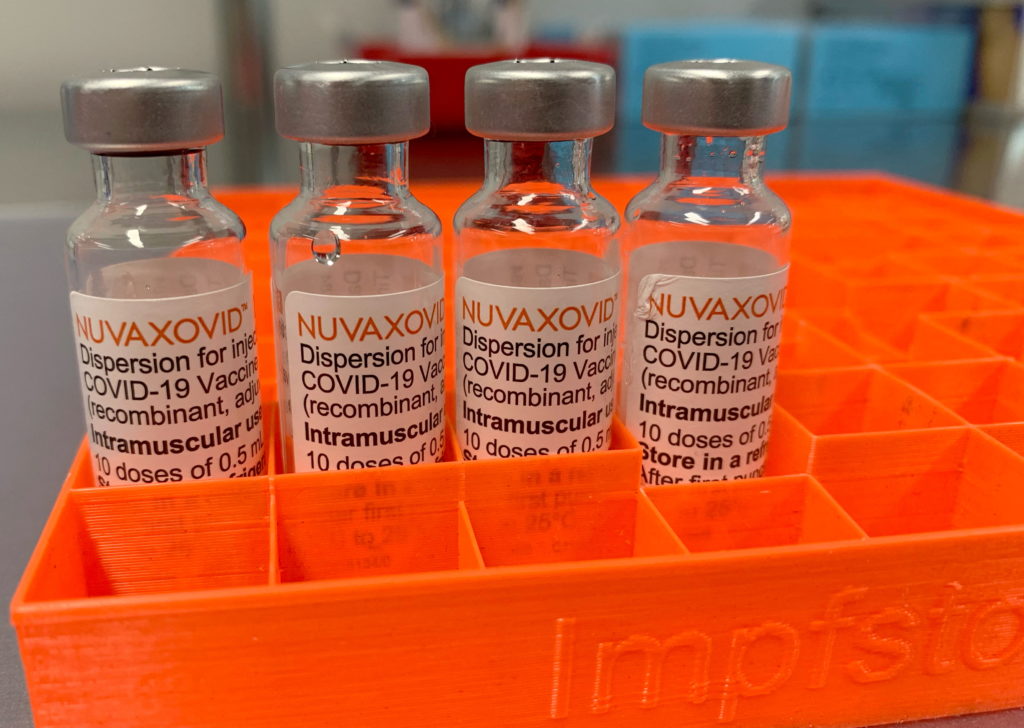
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, has been met with a mix of relief and confusion. While heralded as a welcome addition to the arsenal against the virus, the approval comes with unusual restrictions that have raised eyebrows among health experts and the public alike. This unprecedented approach warrants a closer look.
A New Player in the COVID-19 Vaccine Market
The arrival of Novavax's protein-subunit vaccine, marketed as Nuvaxovid, offered a different approach compared to the mRNA vaccines from Pfizer-BioNTech and Moderna, and the viral vector vaccine from Johnson & Johnson. Many hoped it would address vaccine hesitancy, offering a more traditional vaccine technology that some individuals preferred. However, the FDA's decision to grant full approval with stipulations has cast a shadow on its potential impact.
What are the Unusual Restrictions?
The FDA's approval is not without caveats. Unlike the other authorized COVID-19 vaccines, Nuvaxovid's approval is limited to individuals 18 years of age and older. This is significant, considering that other vaccines have been approved for use in younger age groups. Furthermore, the FDA's authorization explicitly states that Nuvaxovid is not authorized for use as a booster dose.
This limited authorization contrasts sharply with the broader approvals granted to other COVID-19 vaccines. The restrictions significantly limit the potential market and the overall utility of the vaccine.
Why the Restrictions?
The reasons behind these restrictions remain somewhat unclear. While the FDA has not explicitly detailed the rationale, speculation points to factors such as:
- Limited Data: It's possible that the available clinical trial data did not provide sufficient evidence to support authorization for younger age groups or as a booster. Further studies may be needed to ascertain its efficacy and safety in these contexts.
- Manufacturing Challenges: Novavax has faced manufacturing challenges in the past, which may have impacted the data available for review and contributed to the cautious approach taken by the FDA.
- Market Saturation: The landscape of COVID-19 vaccination has significantly changed since the initial development of the Novavax vaccine. The prevalence of highly effective mRNA vaccines and boosters could contribute to a diminished demand for Nuvaxovid.
Implications for Public Health
The restricted approval of the Novavax vaccine raises concerns about its overall impact on public health efforts. The limited availability and specific authorization could hinder its potential to reach vulnerable populations and contribute to broader vaccination rates. This situation underscores the complex dynamics of vaccine development, approval, and deployment.
Looking Ahead
The future of Nuvaxovid remains uncertain. Whether Novavax will pursue further studies to expand the authorization to younger age groups or for booster use remains to be seen. The FDA's decision highlights the rigorous process of vaccine approval and the complexities involved in introducing new vaccines into an already established market. This situation serves as a reminder of the constantly evolving nature of pandemic response and vaccine development. It also highlights the importance of continued surveillance and research to ensure the availability of safe and effective vaccines for all.
Call to Action: Stay informed about the latest COVID-19 vaccine updates from reputable sources like the and the . Consult with your healthcare provider to discuss your vaccination needs and determine the best course of action for your individual circumstances.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine Comes With Unusual Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Wednesday Weather Alert Rain And Cold To Impact Region
May 21, 2025
Wednesday Weather Alert Rain And Cold To Impact Region
May 21, 2025 -
 Nec Tcfd Tnfd Ai
May 21, 2025
Nec Tcfd Tnfd Ai
May 21, 2025 -
 Pro Gamer Uzi Receives Luxurious Electric G Wagon From Mercedes Benz
May 21, 2025
Pro Gamer Uzi Receives Luxurious Electric G Wagon From Mercedes Benz
May 21, 2025 -
 Overnight Storms Expected In Charlotte Prepare For A Temperature Drop
May 21, 2025
Overnight Storms Expected In Charlotte Prepare For A Temperature Drop
May 21, 2025 -
 From Olympic Glory To Emotional Scars A Swimmers Struggle With Coaching Abuse
May 21, 2025
From Olympic Glory To Emotional Scars A Swimmers Struggle With Coaching Abuse
May 21, 2025
Latest Posts
-
 Sesame Streets Move To Netflix Impact Of Lost Trump Funding
May 21, 2025
Sesame Streets Move To Netflix Impact Of Lost Trump Funding
May 21, 2025 -
 Trump Putin Phone Call New Insights Into The Evolving Ukraine Conflict
May 21, 2025
Trump Putin Phone Call New Insights Into The Evolving Ukraine Conflict
May 21, 2025 -
 Temperature Drop Brings Increased Chance Of Showers All Week
May 21, 2025
Temperature Drop Brings Increased Chance Of Showers All Week
May 21, 2025 -
 Widespread Rain And Cold Expected Across The Region Wednesday
May 21, 2025
Widespread Rain And Cold Expected Across The Region Wednesday
May 21, 2025 -
 Ellen De Generes Shares Heartbreaking Post About Irreplaceable Family Loss
May 21, 2025
Ellen De Generes Shares Heartbreaking Post About Irreplaceable Family Loss
May 21, 2025
