FDA Approval For Novavax COVID-19 Vaccine Comes With Unusual Stipulations
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
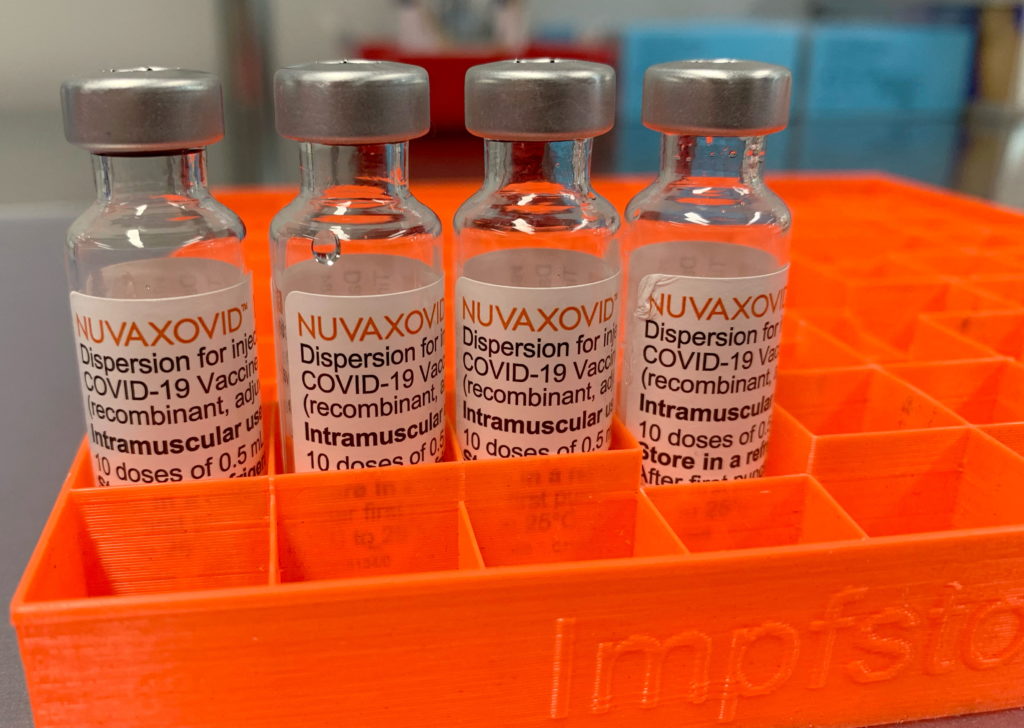
FDA Approval for Novavax COVID-19 Vaccine Comes With Unusual Stipulations
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, has been met with a mix of relief and raised eyebrows. While hailed as a victory for vaccine diversity and potentially boosting vaccination rates, the approval comes with several unusual stipulations that have sparked debate among health experts and the public. This isn't just another vaccine green light; it's a carefully calibrated decision with significant implications.
A New Contender in the COVID-19 Vaccine Market
For months, the Novavax vaccine has been eagerly anticipated. Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, or the viral vector vaccine from Johnson & Johnson, Novavax uses a more traditional protein subunit technology. This approach, utilizing a recombinant spike protein, has been seen by some as a potentially more appealing option for vaccine-hesitant individuals who may have concerns about the newer technologies. The FDA approval seemingly validates this alternative approach, offering a choice for those who prefer it.
The Unusual Stipulations: A Closer Look
However, the FDA's approval isn't without its caveats. The agency has imposed several conditions, making this approval notably different from those granted to other COVID-19 vaccines. These conditions include:
-
Limited Authorized Use: The approval is not for the general population across all age groups. The FDA has specified authorized uses, limiting its application to certain demographics. This targeted approach contrasts with the broader authorizations granted to other vaccines. More information on specific age group limitations can be found on the . Note: This link is for illustrative purposes and may need updating to reflect the most current FDA information.
-
Stringent Manufacturing Oversight: The FDA has implemented rigorous manufacturing oversight, indicating concerns about potential inconsistencies in production. This suggests a need for tighter quality control measures than were initially expected, raising questions about the initial manufacturing process.
-
Ongoing Surveillance: The FDA will maintain a close watch on the vaccine's safety profile post-approval, conducting extensive post-market surveillance. This ongoing monitoring signals a greater level of caution than usually accompanies a vaccine approval.
Why the Unusual Conditions? Expert Opinions Differ
The rationale behind these stipulations remains a subject of discussion. Some experts believe the conditions reflect a cautious approach, given the relatively late entry of Novavax into the already established COVID-19 vaccine market. Others suggest that the conditions might reflect concerns about the vaccine's manufacturing process or initial trial data.
Dr. [Insert Name of relevant expert], a leading immunologist, stated, "The FDA's approach highlights a need for ongoing vigilance in vaccine development and deployment. These stipulations should not be interpreted as a negative reflection on the vaccine's efficacy, but rather as a prudent measure to ensure long-term safety and effectiveness."
Impact on Vaccination Rates and Public Confidence
The impact of these stipulations on vaccination rates remains uncertain. While the availability of a different vaccine type could encourage some hesitant individuals, the specific limitations may also create confusion and potentially limit the vaccine's reach. The FDA's clear communication strategy will be crucial in addressing public concerns and ensuring accurate information dissemination.
Looking Ahead: What's Next for Novavax?
Novavax's journey to market has been long and complex. This approval, despite the unusual stipulations, represents a significant step. The company will need to address the FDA's concerns diligently to ensure smooth distribution and widespread acceptance. The success of Nuvaxovid hinges not only on its efficacy but also on public trust and transparent communication regarding its limitations. The coming months will be crucial in determining the long-term impact of this conditionally approved vaccine.
Call to Action: Stay informed about the latest updates on COVID-19 vaccines by consulting your doctor and reliable sources like the CDC and FDA websites. Understanding the nuances of vaccine approvals is key to making informed healthcare decisions.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine Comes With Unusual Stipulations. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Toledo Mud Hens Defeat Scranton Wilkes Barre Rail Riders In Close Contest
May 20, 2025
Toledo Mud Hens Defeat Scranton Wilkes Barre Rail Riders In Close Contest
May 20, 2025 -
 Freaky Friday Reunion Jamie Lee Curtis Talks Lindsay Lohan Friendship
May 20, 2025
Freaky Friday Reunion Jamie Lee Curtis Talks Lindsay Lohan Friendship
May 20, 2025 -
 Lufthansa Pilotless Flight 10 Minutes Of Unattended Operation After Co Pilot Faint
May 20, 2025
Lufthansa Pilotless Flight 10 Minutes Of Unattended Operation After Co Pilot Faint
May 20, 2025 -
 Helldivers 2 Master Of Ceremony Warbond Drop Details For May 15th
May 20, 2025
Helldivers 2 Master Of Ceremony Warbond Drop Details For May 15th
May 20, 2025 -
 Copyright Lawsuit Hits Australian Body Horror Eurovision Loses Go Jo
May 20, 2025
Copyright Lawsuit Hits Australian Body Horror Eurovision Loses Go Jo
May 20, 2025
Latest Posts
-
 Buy Now Pay Later Overhaul Protecting Consumers From Debt Traps
May 20, 2025
Buy Now Pay Later Overhaul Protecting Consumers From Debt Traps
May 20, 2025 -
 The Us Factory Revealing The Truth About Trumps Economic Policies
May 20, 2025
The Us Factory Revealing The Truth About Trumps Economic Policies
May 20, 2025 -
 Olympic Champions Harrowing Account Of Emotional And Physical Abuse In Elite Sports
May 20, 2025
Olympic Champions Harrowing Account Of Emotional And Physical Abuse In Elite Sports
May 20, 2025 -
 Stock Market Rally S And P 500s 6 Day Winning Streak Dow And Nasdaq Gains
May 20, 2025
Stock Market Rally S And P 500s 6 Day Winning Streak Dow And Nasdaq Gains
May 20, 2025 -
 Espns Untold And Brett Favre A J Perez Recounts Threats And Production Challenges
May 20, 2025
Espns Untold And Brett Favre A J Perez Recounts Threats And Production Challenges
May 20, 2025
