FDA Approval For Novavax COVID-19 Vaccine: Understanding The Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Approval for Novavax COVID-19 Vaccine: Understanding the Restrictions
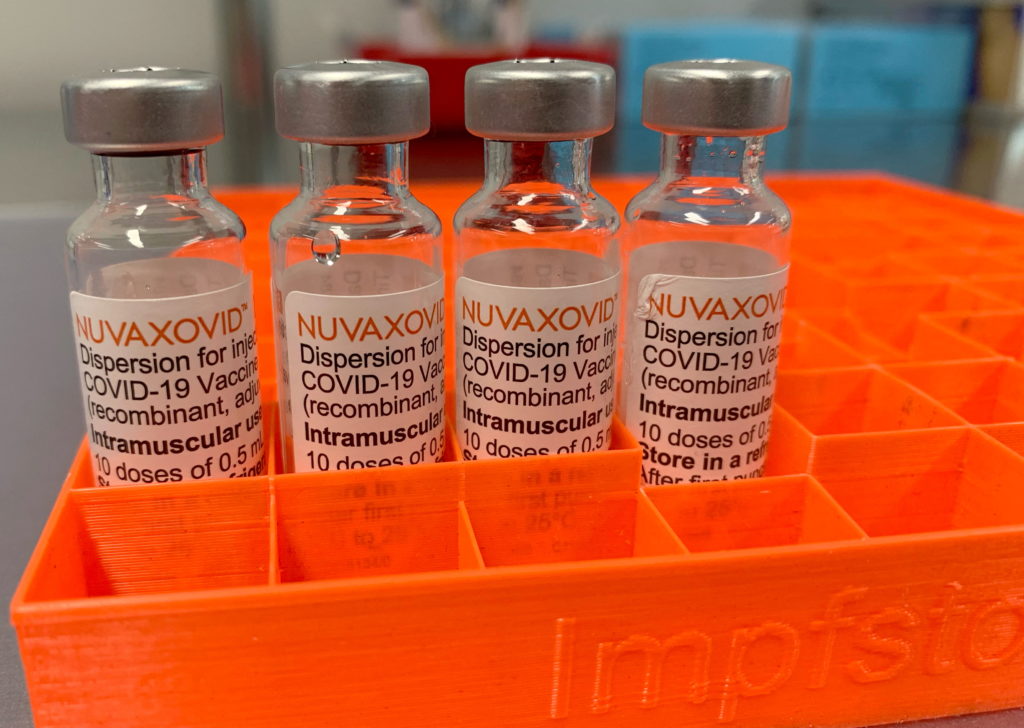
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, marked a significant milestone in the fight against the pandemic. However, this approval isn't a blanket green light. Understanding the nuances of the authorization, including its limitations and target population, is crucial for both healthcare providers and the public. This article delves into the specifics of the FDA's approval, clarifying the restrictions and answering key questions surrounding this new vaccine option.
What is Nuvaxovid and How Does it Differ?
Nuvaxovid, developed by Novavax, is a protein subunit vaccine. Unlike mRNA vaccines like Pfizer-BioNTech and Moderna, it uses a different technology. It employs a lab-made version of the coronavirus spike protein to trigger an immune response. This protein-based approach may appeal to individuals hesitant about mRNA technology, offering an alternative choice within the COVID-19 vaccine landscape. However, it’s important to note that all currently authorized COVID-19 vaccines have proven highly effective in preventing severe illness, hospitalization, and death.
FDA Approval: A Closer Look at the Restrictions
The FDA granted Emergency Use Authorization (EUA) for Nuvaxovid initially, and later transitioned to full licensure for individuals 18 years of age and older. This approval is not for use in children or adolescents. Furthermore, the data supporting the authorization was based on specific trial parameters. While effective, its efficacy might vary depending on emerging variants and individual immune responses.
Who Should Consider Novavax?
- Individuals 18 and older who haven't received a COVID-19 vaccine.
- Those who prefer a protein subunit vaccine over mRNA vaccines.
- People who may have had an adverse reaction to other COVID-19 vaccines (although this should always be discussed with a healthcare provider first).
Limitations and Considerations:
- Age Restrictions: Currently, Nuvaxovid is not approved for use in individuals under 18. Clinical trials for younger age groups are ongoing.
- Variant Effectiveness: While effective against earlier variants, the vaccine's effectiveness against newer variants might differ. Ongoing monitoring and research are crucial to assess its long-term efficacy.
- Side Effects: Like all vaccines, Nuvaxovid can cause side effects. Common side effects include pain at the injection site, fatigue, headache, muscle aches, and nausea. Serious side effects are rare.
- Booster Shots: The FDA's approval doesn't automatically address the need for booster shots. Further research and recommendations regarding boosters will be needed.
Finding Accurate Information:
It's vital to obtain accurate information about Nuvaxovid from reliable sources such as the and the . Be wary of misinformation circulating online.
Conclusion:
The FDA's approval of the Novavax COVID-19 vaccine provides another valuable tool in combating the pandemic. While this approval is a positive development, it’s crucial to understand the existing limitations, particularly the age restrictions and the ongoing need to monitor its efficacy against emerging variants. Always consult with your healthcare provider to determine the best vaccination strategy for your individual circumstances. Staying informed and relying on trustworthy sources are key to making well-informed decisions about your health.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine: Understanding The Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Pachyrhinosaurus Mass Grave A Canadian Paleontological Puzzle
May 20, 2025
Pachyrhinosaurus Mass Grave A Canadian Paleontological Puzzle
May 20, 2025 -
 Did The Ufc Conceal Information About Tom Aspinall Jon Jones Says Yes
May 20, 2025
Did The Ufc Conceal Information About Tom Aspinall Jon Jones Says Yes
May 20, 2025 -
 Analisis La Intensa Semana De Carreras Del Real Madrid
May 20, 2025
Analisis La Intensa Semana De Carreras Del Real Madrid
May 20, 2025 -
 Jamie Lee Curtis Defends Lindsay Lohan A Longstanding Friendship
May 20, 2025
Jamie Lee Curtis Defends Lindsay Lohan A Longstanding Friendship
May 20, 2025 -
 Jamie Lee Curtis And Lindsay Lohan A Candid Friendship Revealed
May 20, 2025
Jamie Lee Curtis And Lindsay Lohan A Candid Friendship Revealed
May 20, 2025
Latest Posts
-
 Down To The Wire Eu And Uk Clash Over Final Brexit Deal Details
May 20, 2025
Down To The Wire Eu And Uk Clash Over Final Brexit Deal Details
May 20, 2025 -
 Enhancing Tourist Safety In Bali A Call For International Collaboration
May 20, 2025
Enhancing Tourist Safety In Bali A Call For International Collaboration
May 20, 2025 -
 How One Us Factory Highlights The Failures Of Trumps Trade Strategy
May 20, 2025
How One Us Factory Highlights The Failures Of Trumps Trade Strategy
May 20, 2025 -
 Balis Plea International Communitys Role In Ensuring Responsible Tourism
May 20, 2025
Balis Plea International Communitys Role In Ensuring Responsible Tourism
May 20, 2025 -
 New Wwi Movie Featuring Daniel Craig Cillian Murphy And Tom Hardy Where To Watch
May 20, 2025
New Wwi Movie Featuring Daniel Craig Cillian Murphy And Tom Hardy Where To Watch
May 20, 2025
