FDA Approves Novavax COVID-19 Vaccine: Understanding The Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Approves Novavax COVID-19 Vaccine: Understanding the Usage Restrictions
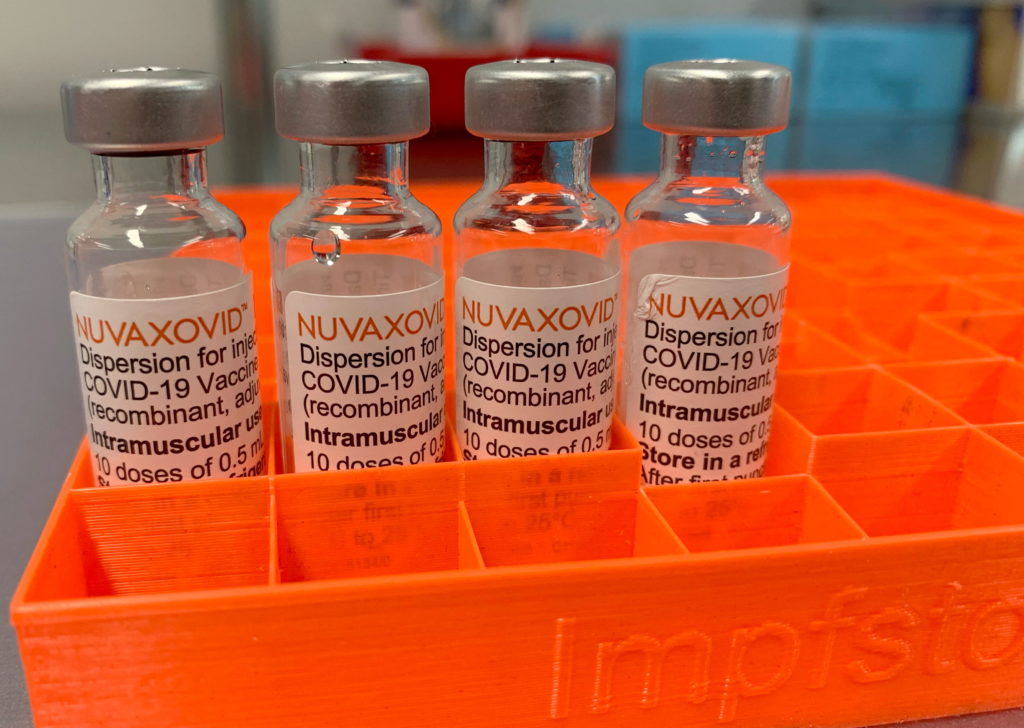
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, marks a significant step in the fight against the pandemic. However, understanding the nuances of its usage restrictions is crucial for both healthcare professionals and the public. This article will delve into the specifics of the approval, highlighting key limitations and answering frequently asked questions.
What is Nuvaxovid?
Nuvaxovid is a protein subunit COVID-19 vaccine. Unlike mRNA vaccines like Pfizer-BioNTech and Moderna, it utilizes a different technology. It presents the body with harmless pieces of the virus's spike protein, prompting an immune response without using the virus's genetic material. This protein-based approach has been a long-standing vaccination strategy, potentially appealing to individuals hesitant about mRNA technology. [Link to CDC page on vaccine types]
FDA Approval and Usage Restrictions:
The FDA granted approval for Nuvaxovid for individuals 18 years of age and older. This is a key restriction, meaning it's currently not authorized for use in children or adolescents. Further research and trials are necessary to determine its safety and efficacy in younger populations.
Another important aspect is the two-dose primary series. The vaccine is administered as two doses, given three to eight weeks apart. [Link to Novavax website for detailed administration guidelines]. Boosters are also authorized, though the specific timing and eligibility criteria may vary depending on individual risk factors and prevailing public health recommendations. Always consult with your healthcare provider to determine the appropriate booster schedule.
Why the Age Restriction?
The age restriction is primarily due to the limited clinical trial data available for individuals younger than 18. While the protein subunit technology is considered generally safe, extensive studies are needed to definitively establish its safety and effectiveness in younger age groups. The FDA prioritizes rigorous testing to ensure the vaccine's efficacy and minimize potential adverse effects in all populations.
Addressing Common Concerns:
Many individuals have questions regarding the safety and efficacy of Nuvaxovid, especially compared to other available COVID-19 vaccines. While clinical trials have shown a robust immune response, it's important to note that no vaccine is 100% effective. Individual responses can vary, and breakthrough infections are possible.
- Efficacy: Nuvaxovid demonstrated high efficacy against symptomatic COVID-19 in clinical trials. However, its effectiveness against newer variants may require further investigation and potentially updated formulations.
- Side Effects: Like other vaccines, Nuvaxovid can cause side effects, such as pain at the injection site, fatigue, headache, and muscle aches. These are generally mild and temporary. Serious side effects are rare. [Link to a credible source on vaccine side effects]
- Allergies: Individuals with known allergies to any component of the vaccine should consult their doctor before receiving the shot.
Conclusion:
The FDA approval of the Novavax COVID-19 vaccine provides another tool in the fight against the pandemic. However, it's crucial to understand the current usage restrictions, particularly the age limitation. This information should empower individuals to make informed decisions about their vaccination choices in consultation with their healthcare providers. Staying up-to-date on the latest information from reliable sources like the CDC and FDA is paramount. Remember, vaccination remains one of the most effective strategies to protect yourself and your community from COVID-19.
Call to Action: Consult your doctor to discuss whether the Novavax vaccine is the right choice for you, considering your individual health circumstances and risk factors.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approves Novavax COVID-19 Vaccine: Understanding The Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Investor Confidence In Ethereum Soars 200 M Investment After Pectra
May 20, 2025
Investor Confidence In Ethereum Soars 200 M Investment After Pectra
May 20, 2025 -
 The Brett Favre Scandal A J Perez On Threats Untolds Future And The Aftermath
May 20, 2025
The Brett Favre Scandal A J Perez On Threats Untolds Future And The Aftermath
May 20, 2025 -
 Espns Untold A J Perez Reveals Favres Camp Threatened Him
May 20, 2025
Espns Untold A J Perez Reveals Favres Camp Threatened Him
May 20, 2025 -
 Addressing Tourist Misconduct Bali Introduces Revised Code Of Conduct
May 20, 2025
Addressing Tourist Misconduct Bali Introduces Revised Code Of Conduct
May 20, 2025 -
 Together Alison Brie And Dave Francos Sundance Film Accused Of Copyright Theft Facing 17 Million Claim
May 20, 2025
Together Alison Brie And Dave Francos Sundance Film Accused Of Copyright Theft Facing 17 Million Claim
May 20, 2025
Latest Posts
-
 The Right Time To Stop Pacifier And Thumb Sucking In Children
May 20, 2025
The Right Time To Stop Pacifier And Thumb Sucking In Children
May 20, 2025 -
 May 15th Brings Masters Of Ceremony Warbond Drops To Helldivers 2
May 20, 2025
May 15th Brings Masters Of Ceremony Warbond Drops To Helldivers 2
May 20, 2025 -
 Justices Alito And Roberts Reflecting On Their Supreme Court Careers
May 20, 2025
Justices Alito And Roberts Reflecting On Their Supreme Court Careers
May 20, 2025 -
 Increased Consumer Protections The Impact Of New Buy Now Pay Later Rules
May 20, 2025
Increased Consumer Protections The Impact Of New Buy Now Pay Later Rules
May 20, 2025 -
 Latest Gaza Hospital Attack Cnn Reports On Israeli Strikes
May 20, 2025
Latest Gaza Hospital Attack Cnn Reports On Israeli Strikes
May 20, 2025
