FDA Approves Novavax COVID-19 Vaccine: Use Restricted
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
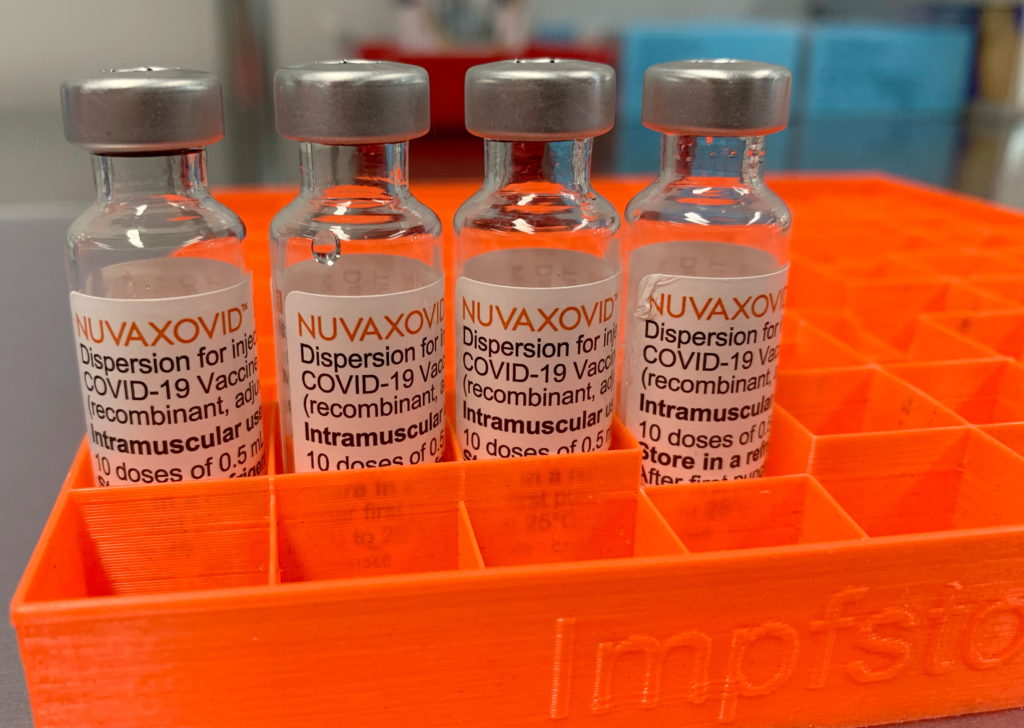
FDA Approves Novavax COVID-19 Vaccine: Use Restricted to Adults
The FDA has granted Emergency Use Authorization (EUA) for the Novavax COVID-19 vaccine, Nuvaxovid, but with a crucial caveat: its use is currently restricted to adults aged 18 years and older. This approval marks a significant milestone in the fight against the pandemic, offering an alternative vaccine option based on a different technology than the mRNA vaccines currently dominating the market. However, the restricted use highlights ongoing considerations regarding its safety and efficacy in younger populations.
A New Contender in the COVID-19 Vaccine Market
Novavax's protein subunit vaccine represents a different approach compared to the mRNA vaccines from Pfizer-BioNTech and Moderna. Instead of using mRNA to instruct cells to produce the virus's spike protein, the Novavax vaccine uses a lab-made version of the spike protein itself. This traditional approach may appeal to individuals hesitant about mRNA technology, although it's important to note that both mRNA and protein subunit vaccines have proven safe and effective in preventing severe COVID-19.
Understanding the FDA's Restricted Authorization
The FDA's decision to grant EUA with age restrictions is a carefully considered one. While the vaccine demonstrated efficacy and a favorable safety profile in adult clinical trials, further data is needed to assess its safety and effectiveness in younger age groups. This is a common practice with new vaccines; rigorous testing across various demographics is essential before widespread rollout. The FDA will continue to monitor data and may expand the EUA to include younger populations in the future, pending further analysis.
What Does this Mean for COVID-19 Vaccination Efforts?
The approval of the Novavax vaccine provides a valuable addition to the arsenal of COVID-19 vaccines. This offers a choice for individuals who may prefer a protein-based vaccine. However, it’s crucial to remember that all currently authorized COVID-19 vaccines are effective at reducing the risk of severe illness, hospitalization, and death. The best vaccine for you depends on individual factors and should be discussed with your healthcare provider.
Addressing Concerns and Misinformation
It’s vital to rely on credible sources of information, such as the FDA website () and the CDC (), when seeking information about COVID-19 vaccines. Misinformation surrounding vaccine safety and efficacy is prevalent, and it's crucial to make informed decisions based on scientific evidence.
Looking Ahead: Expansion of EUA Possible
The FDA’s decision is not a permanent restriction. Novavax will likely conduct further trials to gather data on the vaccine’s efficacy and safety in adolescents and children. Based on the results of these trials, the FDA could expand the EUA to include younger age groups. This underscores the ongoing commitment to thorough research and safety in the development and deployment of COVID-19 vaccines.
Key Takeaways:
- Novavax's COVID-19 vaccine, Nuvaxovid, has received EUA from the FDA.
- Current use is restricted to adults aged 18 and older.
- The vaccine utilizes a protein subunit technology, differing from mRNA vaccines.
- Further trials are needed to assess safety and efficacy in younger age groups.
- Choose a vaccine in consultation with your healthcare provider. All authorized vaccines offer strong protection against severe COVID-19.
This development marks a significant step forward in the global fight against COVID-19, offering another tool to combat the pandemic. The FDA's cautious approach emphasizes the importance of thorough scientific evaluation before widespread vaccine deployment. Stay informed, consult your doctor, and continue practicing preventive measures to protect yourself and your community.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approves Novavax COVID-19 Vaccine: Use Restricted. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Enhancing Tourist Safety And Behavior In Bali A Call For International Assistance
May 20, 2025
Enhancing Tourist Safety And Behavior In Bali A Call For International Assistance
May 20, 2025 -
 Humanitarian Crisis Deepens Israeli Attacks On Gazas Last Hospital
May 20, 2025
Humanitarian Crisis Deepens Israeli Attacks On Gazas Last Hospital
May 20, 2025 -
 Supreme Court Justices Alito And Roberts A Retrospective On Their Long Tenures
May 20, 2025
Supreme Court Justices Alito And Roberts A Retrospective On Their Long Tenures
May 20, 2025 -
 President Biden Receives Prostate Cancer Diagnosis Impact On Presidency
May 20, 2025
President Biden Receives Prostate Cancer Diagnosis Impact On Presidency
May 20, 2025 -
 Behind The Scenes Of Fall Of Favre A Directors Perspective On The Complex Legacy Of Brett Favre
May 20, 2025
Behind The Scenes Of Fall Of Favre A Directors Perspective On The Complex Legacy Of Brett Favre
May 20, 2025
Latest Posts
-
 Solving The Puzzle A Large Pachyrhinosaurus Fossil Site In Canada
May 20, 2025
Solving The Puzzle A Large Pachyrhinosaurus Fossil Site In Canada
May 20, 2025 -
 Bali Tourism Overhaul New Guidelines To Combat Irresponsible Tourist Actions
May 20, 2025
Bali Tourism Overhaul New Guidelines To Combat Irresponsible Tourist Actions
May 20, 2025 -
 Double Tragedy Murders Of Colombian Model And Mexican Influencer Fuel Femicide Debate
May 20, 2025
Double Tragedy Murders Of Colombian Model And Mexican Influencer Fuel Femicide Debate
May 20, 2025 -
 Snl Celebrates 50 Years With Historic Season Finale Episode
May 20, 2025
Snl Celebrates 50 Years With Historic Season Finale Episode
May 20, 2025 -
 Critically Acclaimed Ww 1 Film With Daniel Craig Cillian Murphy And Tom Hardy Available Now
May 20, 2025
Critically Acclaimed Ww 1 Film With Daniel Craig Cillian Murphy And Tom Hardy Available Now
May 20, 2025
