FDA Grants Approval To Novavax COVID-19 Vaccine: Understanding The Conditions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
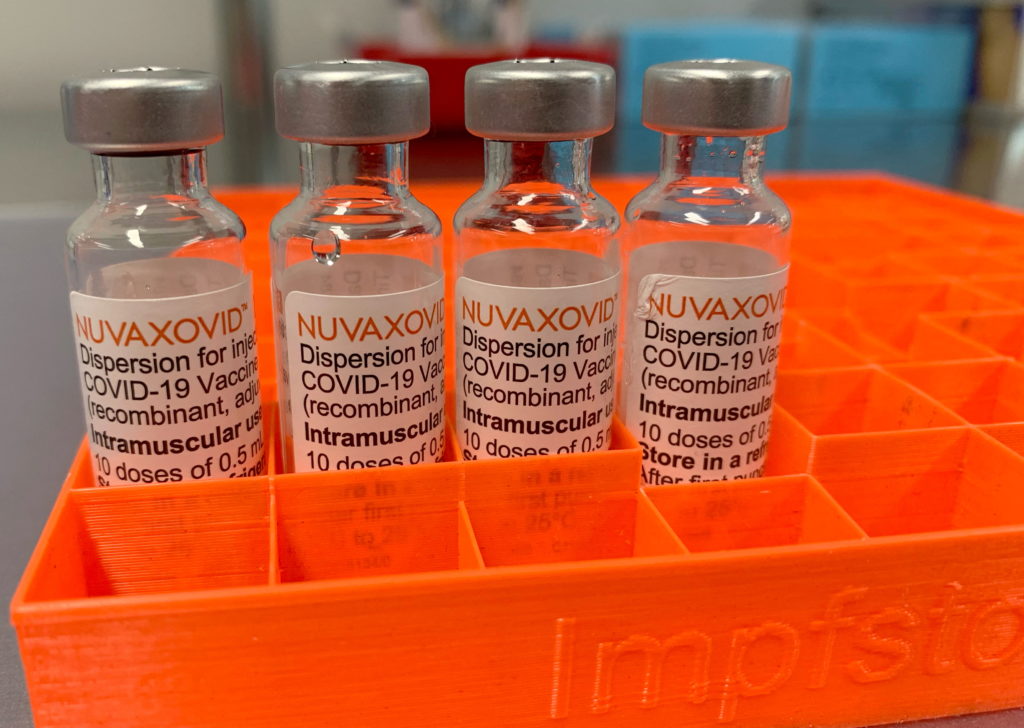
FDA Grants Approval to Novavax COVID-19 Vaccine: Understanding the Conditions
The FDA has granted full approval to the Novavax COVID-19 vaccine, Nuvaxovid, marking a significant milestone in the fight against the pandemic. This approval, however, comes with specific conditions, underscoring the ongoing complexities of vaccine development and deployment. Let's delve into the details of this landmark decision and what it means for public health.
A New Player in the COVID-19 Vaccine Arena
For months, the Novavax vaccine has been available under Emergency Use Authorization (EUA). This full approval, however, represents a heightened level of scrutiny and confidence from the FDA. Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, Novavax utilizes a more traditional protein subunit technology. This technology uses purified fragments of the virus to trigger an immune response, potentially appealing to individuals hesitant about mRNA vaccines. This approval could broaden vaccine access and increase vaccination rates.
Understanding the FDA's Conditions for Approval
While the approval is a victory for Novavax, it's crucial to understand the conditions attached. The FDA's approval is not unconditional. The company is required to:
-
Continue Post-Market Surveillance: Novavax must rigorously monitor the vaccine's safety and effectiveness after its full approval. This ongoing surveillance is a standard procedure for all vaccines, but it's particularly important in the context of a rapidly evolving virus. This includes tracking adverse events and assessing long-term efficacy.
-
Maintain Manufacturing Standards: The FDA will closely scrutinize Novavax's manufacturing processes to ensure consistent quality and safety. Maintaining stringent quality control is paramount to public trust and vaccine effectiveness.
-
Submit Additional Data: The company may be required to submit additional data over time to further solidify the vaccine's safety and efficacy profile. This is an ongoing process and reflects the dynamic nature of scientific research and vaccine development.
What This Means for Individuals
The full approval of the Novavax vaccine offers several potential benefits:
- Increased Confidence: Full FDA approval often translates to increased public trust and may encourage vaccine hesitancy to reconsider vaccination.
- Broader Access: With full approval, the vaccine may become more readily available through various healthcare providers and distribution channels.
- Alternative Option: The protein subunit technology offers an alternative to mRNA vaccines, providing an option for those who prefer this approach.
Addressing Concerns and Misinformation
It's vital to address misinformation surrounding the Novavax vaccine. As with any vaccine, there is a potential for side effects, although serious side effects are rare. The FDA's approval process rigorously evaluates both the benefits and risks. Reliable information about the vaccine's safety and efficacy can be found on the FDA website [link to FDA website] and the CDC website [link to CDC website].
Looking Ahead: The Future of COVID-19 Vaccination
The approval of the Novavax vaccine represents a significant step forward in the global effort to combat COVID-19. While the pandemic continues to evolve, the availability of multiple safe and effective vaccines remains crucial for protecting public health. Continued monitoring, research, and public education will be essential in ensuring the ongoing success of vaccination programs worldwide. Stay informed and consult with your healthcare provider to make informed decisions about your vaccination status.
Call to Action: Learn more about the Novavax vaccine and COVID-19 vaccination from trusted sources like the CDC and FDA websites. Talk to your doctor to determine the best vaccination strategy for you.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Grants Approval To Novavax COVID-19 Vaccine: Understanding The Conditions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Experience The Thrilling Wwi Drama With Daniel Craig Cillian Murphy And Tom Hardy
May 20, 2025
Experience The Thrilling Wwi Drama With Daniel Craig Cillian Murphy And Tom Hardy
May 20, 2025 -
 The Power Of Contrast Comparing The Effectiveness Of Surrender Summit And Post Office Choir Headlines
May 20, 2025
The Power Of Contrast Comparing The Effectiveness Of Surrender Summit And Post Office Choir Headlines
May 20, 2025 -
 Fall Of Favre Director Reveals Challenges In Portraying Brett Favres Complex Story
May 20, 2025
Fall Of Favre Director Reveals Challenges In Portraying Brett Favres Complex Story
May 20, 2025 -
 Snls 50th Season Ends On A High Note Record Breaking Numbers
May 20, 2025
Snls 50th Season Ends On A High Note Record Breaking Numbers
May 20, 2025 -
 May 15th Secure Masters Of Ceremony Warbonds In Helldivers 2
May 20, 2025
May 15th Secure Masters Of Ceremony Warbonds In Helldivers 2
May 20, 2025
Latest Posts
-
 What Is Femicide Examining The Problem And Potential Solutions
May 20, 2025
What Is Femicide Examining The Problem And Potential Solutions
May 20, 2025 -
 Jon Jones Strip The Duck Comment A Deeper Dive Into The Mma World
May 20, 2025
Jon Jones Strip The Duck Comment A Deeper Dive Into The Mma World
May 20, 2025 -
 Misbehaving Tourists Beware Bali Implements New Guidelines
May 20, 2025
Misbehaving Tourists Beware Bali Implements New Guidelines
May 20, 2025 -
 Balis Plea International Collaboration For Safer More Responsible Tourism
May 20, 2025
Balis Plea International Collaboration For Safer More Responsible Tourism
May 20, 2025 -
 New Rules For Buy Now Pay Later Services Enhanced Consumer Safeguards
May 20, 2025
New Rules For Buy Now Pay Later Services Enhanced Consumer Safeguards
May 20, 2025
