FDA Grants Conditional Approval To Novavax COVID-19 Vaccine
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
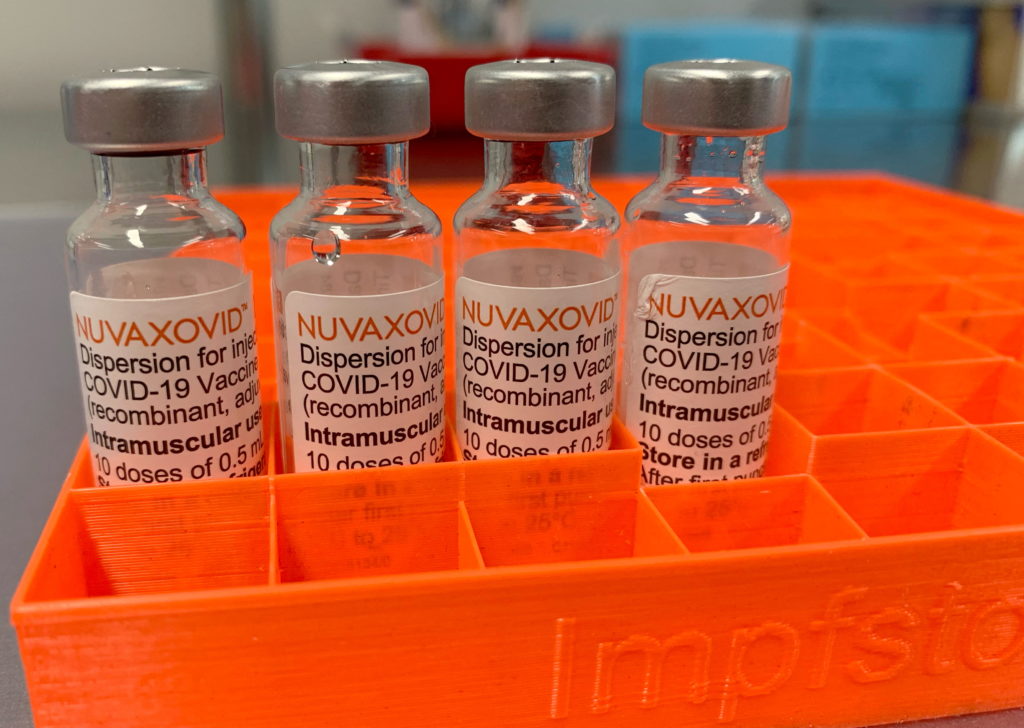
FDA Grants Conditional Approval to Novavax COVID-19 Vaccine: A New Option Arrives
The race for effective COVID-19 vaccines has seen another significant development. On July 13, 2023, the U.S. Food and Drug Administration (FDA) granted Conditional Approval to the Novavax COVID-19 vaccine, Nuvaxovid (NVX-CoV2373). This marks a crucial milestone in the fight against the pandemic, offering individuals hesitant about mRNA vaccines a new alternative. The approval comes after extensive clinical trials demonstrating the vaccine's efficacy and safety profile.
This protein-subunit vaccine, different from the widely used mRNA vaccines like Pfizer-BioNTech and Moderna, utilizes a more traditional approach to vaccine technology. This difference is a key factor for many who have expressed concerns about the novel mRNA technology. The availability of Nuvaxovid provides a welcome choice, potentially broadening vaccination rates among previously hesitant populations.
<h3>Understanding Novavax's Technology: A Different Approach</h3>
Unlike mRNA vaccines, which instruct cells to produce the virus's spike protein, the Novavax vaccine uses purified spike protein antigens. This protein is produced in insect cells and then formulated with Matrix-M™, an adjuvant that enhances the immune response. This established technology may resonate better with individuals seeking a more conventional vaccine option. The use of this adjuvant is noteworthy, as it's designed to strengthen the immune response, potentially leading to greater protection.
This protein-based approach has been used successfully in other vaccines for years, making it a familiar and potentially more reassuring technology for some individuals. This familiarity could be a pivotal factor in increasing vaccination rates globally.
<h3>Efficacy and Safety: What the Data Shows</h3>
The FDA's conditional approval was based on robust clinical trial data demonstrating the vaccine's effectiveness in preventing COVID-19. Studies showed high efficacy against symptomatic disease. While specific efficacy rates can vary based on the study and circulating variants, the overall results are positive and supportive of the vaccine's ability to protect against severe illness.
Importantly, the safety profile of the Novavax vaccine has been thoroughly assessed. Common side effects, such as pain at the injection site, fatigue, headache, and muscle aches, were generally mild and temporary. Serious adverse events were rare. This safety data is crucial in reassuring potential recipients. Further post-market surveillance will continue to monitor the vaccine's long-term safety and efficacy.
<h3>Who Should Get the Novavax Vaccine?</h3>
The Novavax COVID-19 vaccine is authorized for use in individuals 18 years of age and older. It's important to consult with your healthcare provider to determine if this vaccine is the right choice for you. They can assess your individual health status and vaccination history to guide your decision. While the vaccine offers a valuable alternative, it's crucial to make an informed choice based on your specific circumstances.
<h3>The Future of COVID-19 Vaccination</h3>
The conditional approval of the Novavax vaccine is a significant step forward. It provides a much-needed alternative to existing vaccines, potentially addressing hesitancy and increasing overall vaccination rates. The availability of different vaccine types caters to individual preferences and concerns, contributing to a more comprehensive vaccination strategy. The continued monitoring of the vaccine's efficacy against emerging variants will also be crucial in adapting to the evolving pandemic landscape. This new addition to the arsenal of COVID-19 vaccines is a crucial development in the ongoing global effort to protect populations from this virus. For up-to-date information on COVID-19 vaccines, consult the CDC website [link to CDC website].
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Consult with your healthcare provider for any health concerns or before making any decisions related to your health or treatment.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Grants Conditional Approval To Novavax COVID-19 Vaccine. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Joe Bidens Prostate Cancer Diagnosis Impact On Presidency And Public Health
May 20, 2025
Joe Bidens Prostate Cancer Diagnosis Impact On Presidency And Public Health
May 20, 2025 -
 Critically Acclaimed Wwi Drama Starring Daniel Craig Now Available To Stream
May 20, 2025
Critically Acclaimed Wwi Drama Starring Daniel Craig Now Available To Stream
May 20, 2025 -
 Espns Untold Series A J Perez Recounts Favres Intimidation Tactics
May 20, 2025
Espns Untold Series A J Perez Recounts Favres Intimidation Tactics
May 20, 2025 -
 The Hague And Davao Navigating Dutertes Post Election Legal Landscape
May 20, 2025
The Hague And Davao Navigating Dutertes Post Election Legal Landscape
May 20, 2025 -
 Prisoners To Fill Potholes And Clean Streets Under New Government Initiative
May 20, 2025
Prisoners To Fill Potholes And Clean Streets Under New Government Initiative
May 20, 2025
Latest Posts
-
 Jon Jones Cryptic I M Done Message Ufc Career On The Line
May 20, 2025
Jon Jones Cryptic I M Done Message Ufc Career On The Line
May 20, 2025 -
 Family Struck By Train Two Dead One Child Missing Another Hurt
May 20, 2025
Family Struck By Train Two Dead One Child Missing Another Hurt
May 20, 2025 -
 Urgent Search Child Missing After Train Hits Family On Railroad Bridge Killing Two
May 20, 2025
Urgent Search Child Missing After Train Hits Family On Railroad Bridge Killing Two
May 20, 2025 -
 Putins Dismissal Of Trump Highlights Shifting Global Power Dynamics
May 20, 2025
Putins Dismissal Of Trump Highlights Shifting Global Power Dynamics
May 20, 2025 -
 Jamie Lee Curtis On Lindsay Lohan Shes Always Kept It Real
May 20, 2025
Jamie Lee Curtis On Lindsay Lohan Shes Always Kept It Real
May 20, 2025
