FDA Greenlights Novavax COVID-19 Vaccine, But With Significant Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Greenlights Novavax COVID-19 Vaccine, but with Significant Restrictions
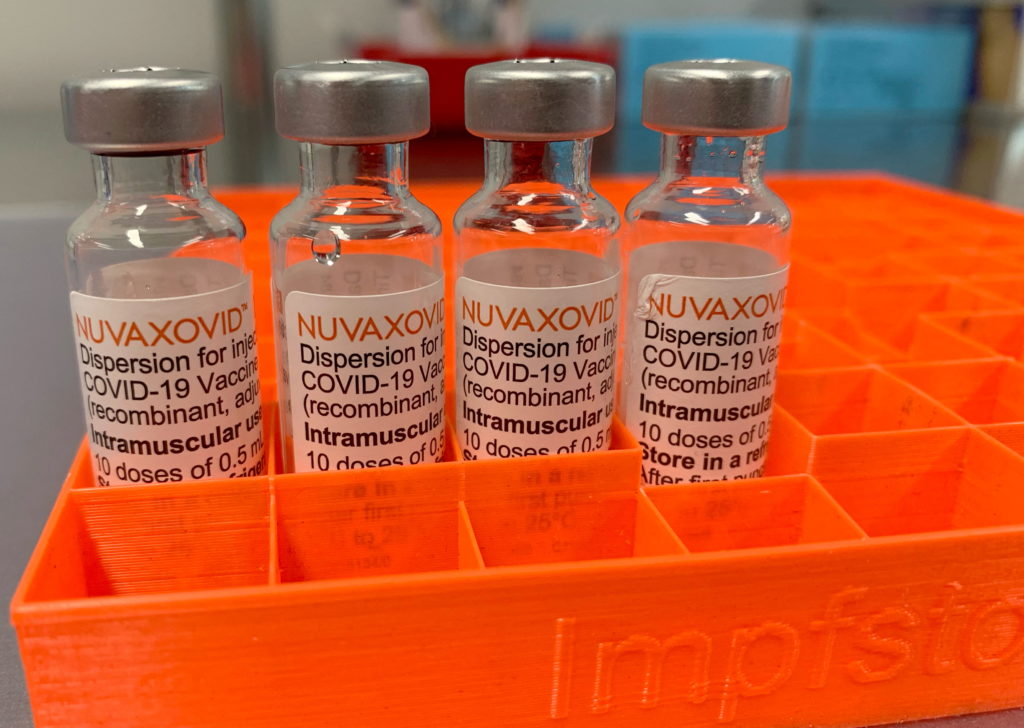
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, has been met with a mixture of relief and cautious optimism. While the authorization marks a significant step in diversifying vaccine options, the accompanying restrictions raise questions about its widespread impact and potential role in the ongoing pandemic. This new vaccine, utilizing a more traditional protein subunit technology, offers a different approach compared to the mRNA vaccines currently dominating the market. However, its limited authorization significantly curtails its immediate availability and potential reach.
A Different Approach: Protein Subunit Technology
Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, which utilize messenger RNA to instruct cells to produce viral proteins, Novavax's Nuvaxovid uses a protein subunit approach. This technology has been around for decades and involves using harmless pieces of the virus to trigger an immune response. This traditional method may appeal to some individuals hesitant about mRNA technology, potentially broadening vaccine uptake among those who remain unvaccinated. However, the FDA's decision to only authorize it under specific conditions highlights inherent challenges.
Significant Restrictions: A Limited Rollout
The FDA's Emergency Use Authorization (EUA) for Nuvaxovid comes with notable restrictions. Firstly, the vaccine is only authorized for individuals aged 18 and older. Secondly, and perhaps most significantly, the authorization is limited to a specific dosage and administration schedule. This contrasts sharply with the more readily available mRNA vaccines, impacting the logistical ease of its distribution and implementation within existing vaccination programs. The FDA’s cautious approach underlines the need for thorough data analysis and monitoring of potential side effects, particularly considering the evolving landscape of COVID-19 variants.
Potential Benefits and Concerns:
The availability of a protein-based vaccine could be beneficial for certain populations who have concerns about mRNA vaccines. For example, individuals with specific pre-existing conditions or those with a history of adverse reactions to other vaccines might find this option more appealing. However, the limited authorization and rollout may hinder its ability to significantly impact overall vaccination rates.
What This Means for the Future:
The FDA’s approval of Novavax's vaccine offers a valuable alternative, but its restricted use raises crucial questions. The limited availability and specific age restrictions may prevent it from becoming a widespread solution. Further research and monitoring are crucial to evaluate its long-term effectiveness and safety profile, especially against emerging COVID-19 variants. The decision underscores the complex challenges involved in vaccine development, distribution, and public acceptance. The long-term impact of Nuvaxovid remains to be seen, but its arrival expands the options available in the fight against COVID-19. The situation will continue to be monitored closely, and updates on its efficacy and availability will be crucial in navigating the ongoing pandemic.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein subunit vaccine, mRNA vaccine, vaccine authorization, emergency use authorization, vaccine restrictions, vaccination rates, COVID-19 variants, vaccine safety, vaccine efficacy.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Greenlights Novavax COVID-19 Vaccine, But With Significant Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Future Of Match Of The Day Uncertain After Gary Linekers Expected Bbc Exit
May 21, 2025
Future Of Match Of The Day Uncertain After Gary Linekers Expected Bbc Exit
May 21, 2025 -
 Years Of Waiting End Hostage Returns Home Family Shares Emotional Story
May 21, 2025
Years Of Waiting End Hostage Returns Home Family Shares Emotional Story
May 21, 2025 -
 New Peaky Blinders Series Officially Confirmed Expect The Unexpected
May 21, 2025
New Peaky Blinders Series Officially Confirmed Expect The Unexpected
May 21, 2025 -
 Did The Ufc Withhold Key Information On Tom Aspinall Jones Demands Answers
May 21, 2025
Did The Ufc Withhold Key Information On Tom Aspinall Jones Demands Answers
May 21, 2025 -
 New Rayman Game Ubisoft Milan Seeks Experienced Developers
May 21, 2025
New Rayman Game Ubisoft Milan Seeks Experienced Developers
May 21, 2025
Latest Posts
-
 Rain And Chilly Temperatures Sweep Across Region Wednesday
May 21, 2025
Rain And Chilly Temperatures Sweep Across Region Wednesday
May 21, 2025 -
 Irreplaceable Ellen De Generes Emotional Tribute Following Family Death
May 21, 2025
Irreplaceable Ellen De Generes Emotional Tribute Following Family Death
May 21, 2025 -
 Dork Politicians Tim Dillons Candid Cnn Interview Sparks Debate
May 21, 2025
Dork Politicians Tim Dillons Candid Cnn Interview Sparks Debate
May 21, 2025 -
 Abandoned Chicks Delaware Shelter Seeks Help After Usps Delivery Disaster
May 21, 2025
Abandoned Chicks Delaware Shelter Seeks Help After Usps Delivery Disaster
May 21, 2025 -
 Outrage As New Law Impacts Paedophiles Parental Rights Family Speaks Out
May 21, 2025
Outrage As New Law Impacts Paedophiles Parental Rights Family Speaks Out
May 21, 2025
