FDA Greenlights Novavax COVID-19 Vaccine, Imposes Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Greenlights Novavax COVID-19 Vaccine, But with Usage Restrictions
The FDA has approved a second protein-based COVID-19 vaccine, offering an alternative for those hesitant about mRNA technology. However, the authorization comes with limitations.
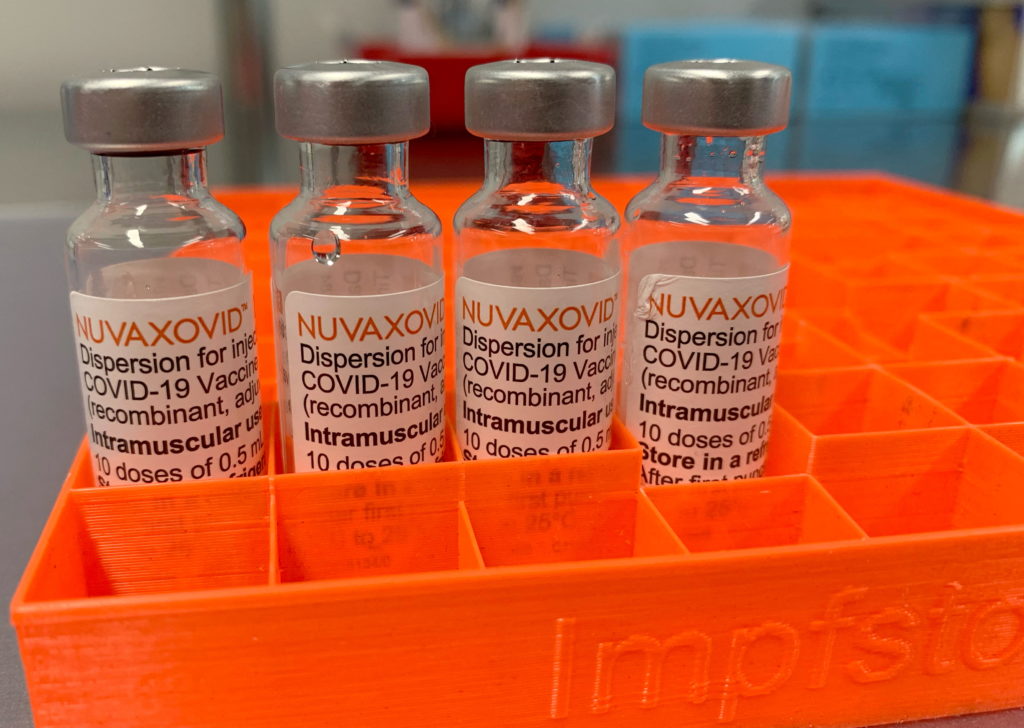
The U.S. Food and Drug Administration (FDA) has granted Emergency Use Authorization (EUA) to Novavax's COVID-19 vaccine, Nuvaxovid, marking a significant development in the fight against the pandemic. This approval introduces a new option for individuals seeking vaccination, particularly those who may have been hesitant to receive mRNA vaccines like Pfizer-BioNTech and Moderna. The vaccine, a protein-subunit vaccine, uses a different technology than its mRNA counterparts, potentially appealing to a broader segment of the population.
However, the FDA's authorization isn't without caveats. The agency has imposed specific usage restrictions, limiting its application to certain groups and highlighting potential side effects. Understanding these limitations is crucial for both healthcare providers and individuals considering this vaccine.
<h3>Understanding Novavax's Nuvaxovid: A Different Approach</h3>
Unlike mRNA vaccines, which utilize messenger RNA to instruct cells to produce viral proteins, Nuvaxovid uses a more traditional approach. It leverages a harmless version of the spike protein found on the surface of the SARS-CoV-2 virus. This protein triggers an immune response, preparing the body to fight off the actual virus. This technology has been used in other vaccines for decades, potentially alleviating concerns some individuals have about the newer mRNA technology.
This difference in technology is a key factor driving interest in Nuvaxovid. For individuals wary of mRNA vaccines, this protein-based option may provide a more comfortable alternative. This broadened vaccine access could be crucial in achieving higher vaccination rates globally.
<h3>FDA's Usage Restrictions: What You Need to Know</h3>
While the FDA's approval is positive news, the agency has implemented specific restrictions:
- Age Limitations: Currently, Nuvaxovid is authorized for individuals 18 years of age and older. Further trials are needed to assess its safety and efficacy in younger populations.
- Dosage: The vaccine is administered as a two-dose primary series, given three to eight weeks apart.
- Potential Side Effects: Common side effects reported in clinical trials include pain at the injection site, fatigue, headache, muscle aches, and nausea. More serious side effects are rare but possible. It's essential to consult with a healthcare provider if you experience any concerning symptoms.
- Limited Data on Variants: While effective against the original COVID-19 strain, data on its efficacy against newer variants is still being gathered and analyzed. This is an ongoing area of research for all COVID-19 vaccines.
<h3>What This Means for the Future of COVID-19 Vaccination</h3>
The FDA's approval of Novavax's vaccine is a significant step towards broader vaccine access and potentially higher vaccination rates. Offering a different vaccine technology provides choice and addresses concerns some individuals have about mRNA vaccines. However, it's crucial to remember that the usage restrictions are in place for safety reasons. Individuals considering Nuvaxovid should discuss the risks and benefits with their healthcare provider to make an informed decision. The ongoing evaluation of the vaccine's efficacy against emerging variants remains a key area of focus for ongoing research. Stay informed about updates from reputable sources like the CDC and FDA.
Call to Action: Consult with your doctor to determine which COVID-19 vaccine is right for you. Remember, vaccination remains a crucial tool in protecting yourself and your community against COVID-19. For more information, visit the and the .

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Greenlights Novavax COVID-19 Vaccine, Imposes Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Down To The Wire Eu Uk Brexit Negotiations Face Impasse
May 21, 2025
Down To The Wire Eu Uk Brexit Negotiations Face Impasse
May 21, 2025 -
 Two Teens Face Charges After Defecating And Urinating At Santa Rosa Church
May 21, 2025
Two Teens Face Charges After Defecating And Urinating At Santa Rosa Church
May 21, 2025 -
 One And Done Feds 2025 Rate Cut Projection Impacts U S Treasury Yields
May 21, 2025
One And Done Feds 2025 Rate Cut Projection Impacts U S Treasury Yields
May 21, 2025 -
 Alito And Roberts Near 30 Years On The Supreme Court An Analysis
May 21, 2025
Alito And Roberts Near 30 Years On The Supreme Court An Analysis
May 21, 2025 -
 Mma World Reacts Jon Jones Controversial Strip The Duck Comment On Tom Aspinall
May 21, 2025
Mma World Reacts Jon Jones Controversial Strip The Duck Comment On Tom Aspinall
May 21, 2025
Latest Posts
-
 Police Investigate Desecration Of Santa Rosa Church Teenagers Involved
May 21, 2025
Police Investigate Desecration Of Santa Rosa Church Teenagers Involved
May 21, 2025 -
 Near Collision At La Guardia Prompts Joint Faa And Ntsb Inquiry
May 21, 2025
Near Collision At La Guardia Prompts Joint Faa And Ntsb Inquiry
May 21, 2025 -
 Rain Cold Temperatures Expected Across Region Wednesday
May 21, 2025
Rain Cold Temperatures Expected Across Region Wednesday
May 21, 2025 -
 Ubisoft Explains Animal Killing Restrictions In Assassins Creed Valhalla
May 21, 2025
Ubisoft Explains Animal Killing Restrictions In Assassins Creed Valhalla
May 21, 2025 -
 World Press Photo Re Examines Attribution Of Iconic Napalm Girl Vietnam War Image
May 21, 2025
World Press Photo Re Examines Attribution Of Iconic Napalm Girl Vietnam War Image
May 21, 2025
