FDA Greenlights Novavax COVID-19 Vaccine With Conditional Authorization
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
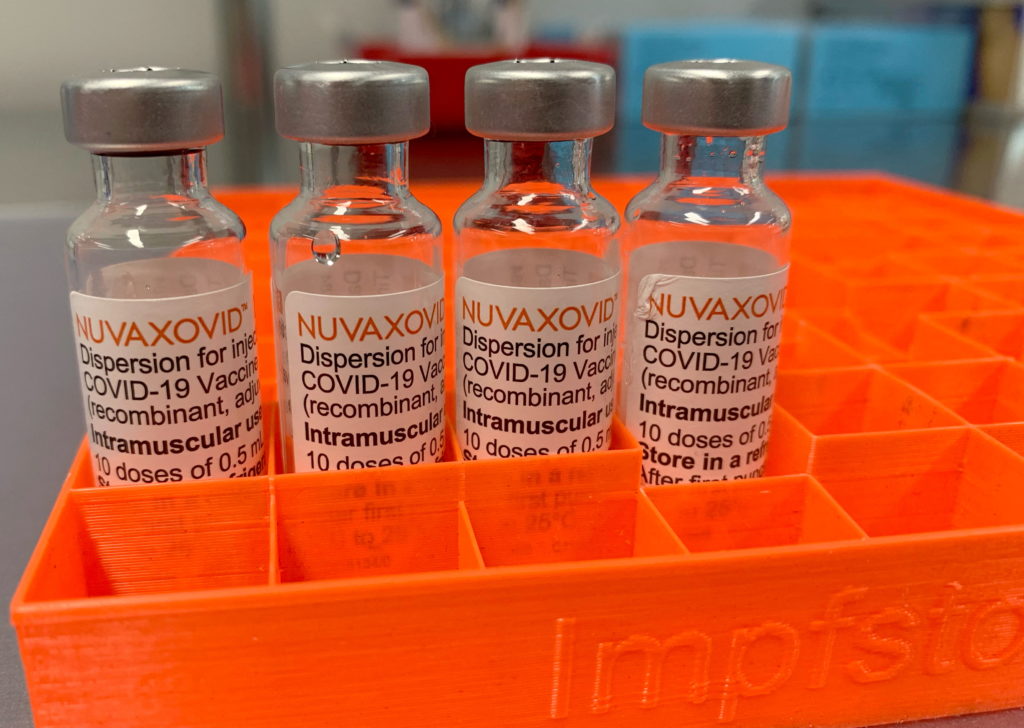
FDA Greenlights Novavax COVID-19 Vaccine: A New Option Arrives
The FDA's conditional authorization of the Novavax COVID-19 vaccine marks a significant step in the fight against the pandemic. This new vaccine offers a different approach compared to the mRNA vaccines already available, providing an alternative for those hesitant to receive the Pfizer-BioNTech or Moderna shots. This decision could significantly impact vaccination rates and broaden access to crucial COVID-19 protection.
What Makes Novavax Different?
Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, which use messenger RNA to instruct cells to produce a harmless piece of the virus, Novavax employs a more traditional protein subunit technology. This technology uses a lab-grown version of the coronavirus spike protein to trigger an immune response. This protein-based approach may appeal to individuals with concerns about mRNA technology, offering a familiar vaccination method with a proven safety track record.
The FDA's Conditional Authorization: What it Means
The FDA's conditional approval is not a full approval, but it signifies that the vaccine has met rigorous safety and efficacy standards. This authorization allows for the widespread distribution and use of the vaccine while ongoing monitoring and data collection continue. The FDA will continue to evaluate the long-term safety and efficacy of the Novavax vaccine.
Addressing Vaccine Hesitancy:
The arrival of the Novavax vaccine could be a game-changer in addressing vaccine hesitancy. For individuals who have been hesitant due to concerns about mRNA technology or its relatively rapid development, this protein-subunit vaccine presents a viable alternative. This could encourage wider vaccine uptake and contribute to increased herd immunity, offering better protection for the entire population.
Boosting Vaccination Rates:
The availability of multiple COVID-19 vaccine options is vital for achieving widespread vaccination coverage. The Novavax vaccine adds another tool to the arsenal, potentially increasing the number of people willing to get vaccinated and strengthening community-wide protection against the virus. This expanded choice is expected to particularly benefit individuals who may have previously been hesitant due to concerns about the existing vaccines.
Looking Ahead: Continued Monitoring and Efficacy
While this is a positive step forward, it's crucial to emphasize that the FDA's conditional approval involves ongoing monitoring. The agency will continue to collect data on the vaccine's long-term safety and efficacy, ensuring the ongoing safety of the public. Further studies are crucial to fully understand its long-term effectiveness and its role in protecting against emerging variants.
Conclusion: A Welcome Addition to the COVID-19 Vaccine Arsenal
The FDA's authorization of the Novavax COVID-19 vaccine is a significant development in the fight against COVID-19. By offering a different vaccine technology, it addresses concerns of some individuals hesitant about receiving the currently available vaccines, potentially boosting vaccination rates and overall community immunity. This development marks a positive step towards a more protected and healthier future. However, continued vigilance and monitoring of the vaccine's long-term effects remain essential. For the latest information on COVID-19 vaccines, consult the .

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Greenlights Novavax COVID-19 Vaccine With Conditional Authorization. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 The Contradictions Of Trumps Trade Policies Evidence From A Us Manufacturing Plant
May 21, 2025
The Contradictions Of Trumps Trade Policies Evidence From A Us Manufacturing Plant
May 21, 2025 -
 Investors Bet Big On Ethereum 200 M Investment After Pectra
May 21, 2025
Investors Bet Big On Ethereum 200 M Investment After Pectra
May 21, 2025 -
 Espns Untold A J Perez Recounts Intimidation In Favre Investigation
May 21, 2025
Espns Untold A J Perez Recounts Intimidation In Favre Investigation
May 21, 2025 -
 Cnn Reports Israeli Strikes Hit Only Remaining Hospital In Northern Gaza
May 21, 2025
Cnn Reports Israeli Strikes Hit Only Remaining Hospital In Northern Gaza
May 21, 2025 -
 Ufc Accused By Jon Jones Of Withholding Aspinall Fight Information
May 21, 2025
Ufc Accused By Jon Jones Of Withholding Aspinall Fight Information
May 21, 2025
Latest Posts
-
 Harsh Coaching Weight Criticism An Olympic Gold Medalists Struggle For Recovery
May 21, 2025
Harsh Coaching Weight Criticism An Olympic Gold Medalists Struggle For Recovery
May 21, 2025 -
 Death Toll Rises After Israeli Attack On Last North Gaza Hospital
May 21, 2025
Death Toll Rises After Israeli Attack On Last North Gaza Hospital
May 21, 2025 -
 Novavax Covid 19 Vaccine Fda Approval Comes With Unexpected Usage Constraints
May 21, 2025
Novavax Covid 19 Vaccine Fda Approval Comes With Unexpected Usage Constraints
May 21, 2025 -
 Prostate Cancer Diagnosis For President Joe Biden Implications And Response
May 21, 2025
Prostate Cancer Diagnosis For President Joe Biden Implications And Response
May 21, 2025 -
 Tom Aspinall Contract Talks And Jon Jones Retirement Hint Whats Next For The Ufc
May 21, 2025
Tom Aspinall Contract Talks And Jon Jones Retirement Hint Whats Next For The Ufc
May 21, 2025
