FDA's Conditional Approval Of Novavax COVID-19 Vaccine: What You Need To Know
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
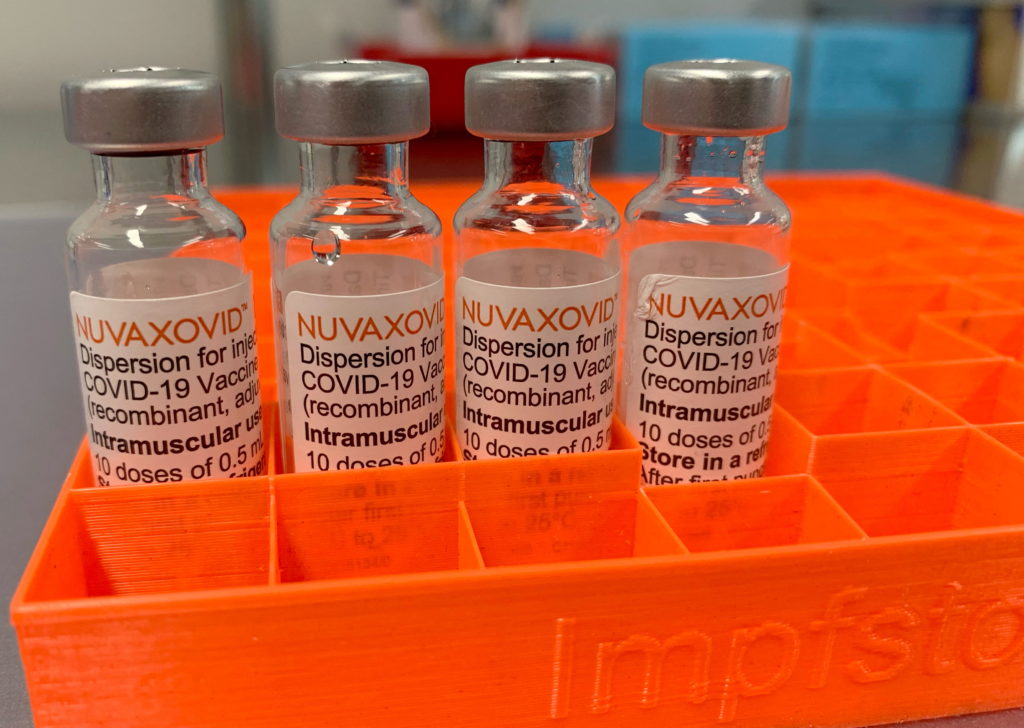
FDA's Conditional Approval of Novavax COVID-19 Vaccine: What You Need to Know
The FDA's conditional approval of the Novavax COVID-19 vaccine marks a significant development in the fight against the pandemic. This protein-subunit vaccine offers a different approach compared to the mRNA vaccines from Pfizer-BioNTech and Moderna, providing a crucial alternative for individuals hesitant to receive mRNA-based injections. But what exactly does this approval mean, and what should you know before considering this vaccine?
Understanding the Novavax Vaccine: A Protein-Subunit Approach
Unlike mRNA vaccines that utilize messenger RNA to instruct cells to produce a viral protein, the Novavax vaccine uses a more traditional method. It contains purified fragments of the SARS-CoV-2 virus spike protein, combined with an adjuvant to enhance the immune response. This protein-subunit approach has been used successfully in other vaccines for decades, potentially addressing concerns some people have about the newer mRNA technology. This makes the Novavax vaccine a compelling option for those seeking a different vaccination pathway.
Key Differences from mRNA Vaccines:
- Technology: Novavax uses a proven protein-subunit technology, while Pfizer-BioNTech and Moderna utilize mRNA technology.
- Storage: Novavax boasts a more straightforward cold chain storage requirement compared to the ultra-low temperature needs of some mRNA vaccines, potentially improving accessibility in diverse settings.
- Side Effects: While side effects vary from person to person, early trials suggest Novavax may have a slightly different side effect profile compared to mRNA vaccines. More data is needed for a complete comparison.
FDA Approval and Safety Considerations
The FDA's conditional approval wasn't a simple green light. It followed rigorous testing and evaluation of the vaccine's safety and efficacy. The agency assessed data from clinical trials demonstrating the vaccine's effectiveness in preventing COVID-19, particularly severe illness. However, like all vaccines, Novavax carries potential side effects. Common side effects reported include pain at the injection site, fatigue, headache, muscle aches, and joint pain. Serious side effects are rare.
It's crucial to consult your doctor before receiving any vaccine, including the Novavax vaccine. They can assess your individual health status and determine if the Novavax COVID-19 vaccine is the right choice for you.
Novavax's Role in the Ongoing Pandemic Response
The addition of the Novavax vaccine to the arsenal of available COVID-19 vaccines is a positive development. It offers a valuable alternative for individuals who may have hesitated to receive mRNA vaccines due to concerns about the technology or potential side effects. This increased choice can potentially lead to higher overall vaccination rates, contributing significantly to herd immunity and a reduction in severe COVID-19 cases.
Where to Get the Novavax Vaccine
The availability of the Novavax vaccine will vary depending on your location. Check with your healthcare provider or local health department for information on vaccine availability and scheduling appointments. You can also consult resources like the for up-to-date information on vaccine distribution.
Looking Ahead: Continued Monitoring and Research
The FDA's approval is conditional, meaning ongoing monitoring and further data collection will continue. This ensures continued assessment of the vaccine's long-term safety and efficacy. Further research will also help refine our understanding of the vaccine's performance against emerging variants of the SARS-CoV-2 virus.
Disclaimer: This article provides general information and should not be considered medical advice. Consult with a healthcare professional for any health concerns or before making any decisions related to your health or treatment.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA's Conditional Approval Of Novavax COVID-19 Vaccine: What You Need To Know. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Bali Tourism Overhaul New Guidelines To Combat Irresponsible Tourist Actions
May 20, 2025
Bali Tourism Overhaul New Guidelines To Combat Irresponsible Tourist Actions
May 20, 2025 -
 Aussie Horror Film In Copyright Dispute Eurovisions Go Jo Eliminated
May 20, 2025
Aussie Horror Film In Copyright Dispute Eurovisions Go Jo Eliminated
May 20, 2025 -
 The Price Of Gold Olympic Swimmers Struggle With Coaching Pressure And Body Image
May 20, 2025
The Price Of Gold Olympic Swimmers Struggle With Coaching Pressure And Body Image
May 20, 2025 -
 Major Data Breach At Legal Aid Client Criminal Records Compromised
May 20, 2025
Major Data Breach At Legal Aid Client Criminal Records Compromised
May 20, 2025 -
 Brett Favre Sexting Scandal Jenn Sterger Details The Emotional Toll
May 20, 2025
Brett Favre Sexting Scandal Jenn Sterger Details The Emotional Toll
May 20, 2025
Latest Posts
-
 Weaning Your Child From Pacifiers And Thumb Sucking Challenges And Solutions
May 20, 2025
Weaning Your Child From Pacifiers And Thumb Sucking Challenges And Solutions
May 20, 2025 -
 Critically Acclaimed Wwi Film With Daniel Craig Cillian Murphy And Tom Hardy Streaming Today
May 20, 2025
Critically Acclaimed Wwi Film With Daniel Craig Cillian Murphy And Tom Hardy Streaming Today
May 20, 2025 -
 Lufthansa Flight Operated Without Pilot For 10 Minutes Details Emerge In New Report
May 20, 2025
Lufthansa Flight Operated Without Pilot For 10 Minutes Details Emerge In New Report
May 20, 2025 -
 Bali Seeks International Cooperation To Improve Tourist Behavior
May 20, 2025
Bali Seeks International Cooperation To Improve Tourist Behavior
May 20, 2025 -
 Lufthansa Plane Flies Unaided For 10 Minutes Following Co Pilots Medical Emergency
May 20, 2025
Lufthansa Plane Flies Unaided For 10 Minutes Following Co Pilots Medical Emergency
May 20, 2025
