Limited FDA Approval: Understanding The Novavax COVID-19 Vaccine Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Limited FDA Approval: Understanding the Novavax COVID-19 Vaccine Restrictions
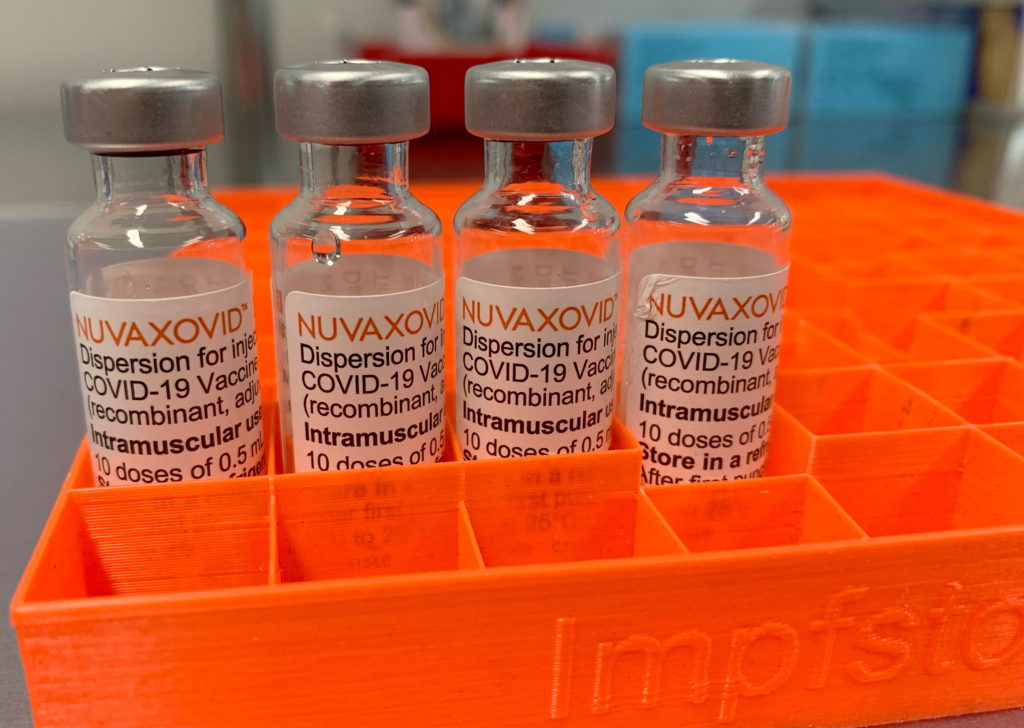
The Novavax COVID-19 vaccine, marketed as Nuvaxovid, has received limited FDA approval, sparking questions and concerns among the public. While its arrival offered an alternative to mRNA vaccines, understanding the nuances of its approval is crucial for informed decision-making. This article will delve into the specifics of the FDA's authorization, highlighting its limitations and answering frequently asked questions.
What is the FDA's Approval Status for the Novavax Vaccine?
The Novavax COVID-19 vaccine received Emergency Use Authorization (EUA) from the FDA in July 2022. Crucially, this is not full FDA approval. While the EUA allowed for its widespread use during the public health emergency, it differed significantly from a full Biologics License Application (BLA) approval. The difference lies primarily in the extent of long-term safety and efficacy data required. EUAs, by their nature, expedite the process based on available evidence, while BLAs demand more extensive and rigorous testing. Recently, Novavax did receive a full BLA for use in individuals 18 years of age and older, but the approval remains limited compared to other widely used vaccines.
What are the Key Restrictions of the Novavax Vaccine Approval?
While approved for use in adults, several factors limit its widespread adoption compared to Pfizer-BioNTech and Moderna vaccines:
- Age Restrictions: Initially, the EUA and the current BLA only cover adults. Approval for younger age groups is still pending further clinical trials and FDA review. This significantly reduces its potential market reach.
- Efficacy Concerns: While the Novavax vaccine demonstrated efficacy against COVID-19 in clinical trials, its effectiveness might be slightly lower compared to mRNA vaccines, particularly against certain variants. Ongoing monitoring and data analysis are crucial to assessing its long-term efficacy. This is a key factor contributing to the limited uptake.
- Logistical Challenges: The Novavax vaccine requires specific storage and handling conditions, potentially adding logistical hurdles to distribution and vaccination campaigns, particularly in resource-constrained settings. This complexity can limit accessibility.
- Public Perception: Following the initial rollout of mRNA vaccines, some hesitancy and vaccine skepticism may have contributed to slower adoption of the Novavax vaccine. Addressing public concerns through transparent communication and education is essential for increasing uptake.
Why the Limited Approval?
The FDA's cautious approach stems from a commitment to rigorous safety and efficacy standards. Although the Novavax vaccine showed promise, the available data at the time of EUA and even now with the BLA fell short of the extensive long-term data needed for full approval across all age groups. The FDA's phased approach reflects a commitment to thorough evaluation before granting unrestricted approval.
What Does the Future Hold for the Novavax Vaccine?
Novavax continues to conduct clinical trials to expand the approval to include younger populations and gather more long-term safety data. The results of these trials will be crucial in determining whether the FDA grants full approval without restrictions. Further research on the vaccine’s effectiveness against emerging variants is also underway.
Conclusion:
The limited FDA approval of the Novavax COVID-19 vaccine reflects a careful, data-driven approach to ensuring public safety. While offering an alternative vaccine technology, understanding the restrictions surrounding its use is vital for both healthcare professionals and the public. Continued monitoring and further research will play a critical role in shaping the future of this vaccine and its role in the ongoing fight against COVID-19. Stay informed about the latest updates from the FDA and your healthcare provider to make informed decisions about your vaccination strategy.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Limited FDA Approval: Understanding The Novavax COVID-19 Vaccine Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Balancing Act The Director Of Netflixs Fall Of Favre Discusses The Documentary
May 20, 2025
Balancing Act The Director Of Netflixs Fall Of Favre Discusses The Documentary
May 20, 2025 -
 President Bidens Health Understanding Prostate Cancer And Its Implications
May 20, 2025
President Bidens Health Understanding Prostate Cancer And Its Implications
May 20, 2025 -
 The Putin Trump Dynamic A Shift In Global Power
May 20, 2025
The Putin Trump Dynamic A Shift In Global Power
May 20, 2025 -
 Jon Jones Ufc Withheld Crucial Aspinall Injury Update
May 20, 2025
Jon Jones Ufc Withheld Crucial Aspinall Injury Update
May 20, 2025 -
 Liranzos Homer Highlights Eries Loss To High Scoring Mud Hens
May 20, 2025
Liranzos Homer Highlights Eries Loss To High Scoring Mud Hens
May 20, 2025
Latest Posts
-
 The Favre Scandal A J Perez On Intimidation And The Espn Documentary
May 21, 2025
The Favre Scandal A J Perez On Intimidation And The Espn Documentary
May 21, 2025 -
 Femicide In The Spotlight Recent Murders Of A Colombian Model And Mexican Influencer Demand Action
May 21, 2025
Femicide In The Spotlight Recent Murders Of A Colombian Model And Mexican Influencer Demand Action
May 21, 2025 -
 Joe Biden Prostate Cancer Diagnosis Implications For Presidency
May 21, 2025
Joe Biden Prostate Cancer Diagnosis Implications For Presidency
May 21, 2025 -
 I M Done Jon Jones Cryptic Message Fuels Ufc Retirement Rumors
May 21, 2025
I M Done Jon Jones Cryptic Message Fuels Ufc Retirement Rumors
May 21, 2025 -
 Investigation Underway After Train Kills Two Injures Children On Bridge
May 21, 2025
Investigation Underway After Train Kills Two Injures Children On Bridge
May 21, 2025
