Novavax COVID-19 Vaccine Approved By FDA With Unusual Limitations
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
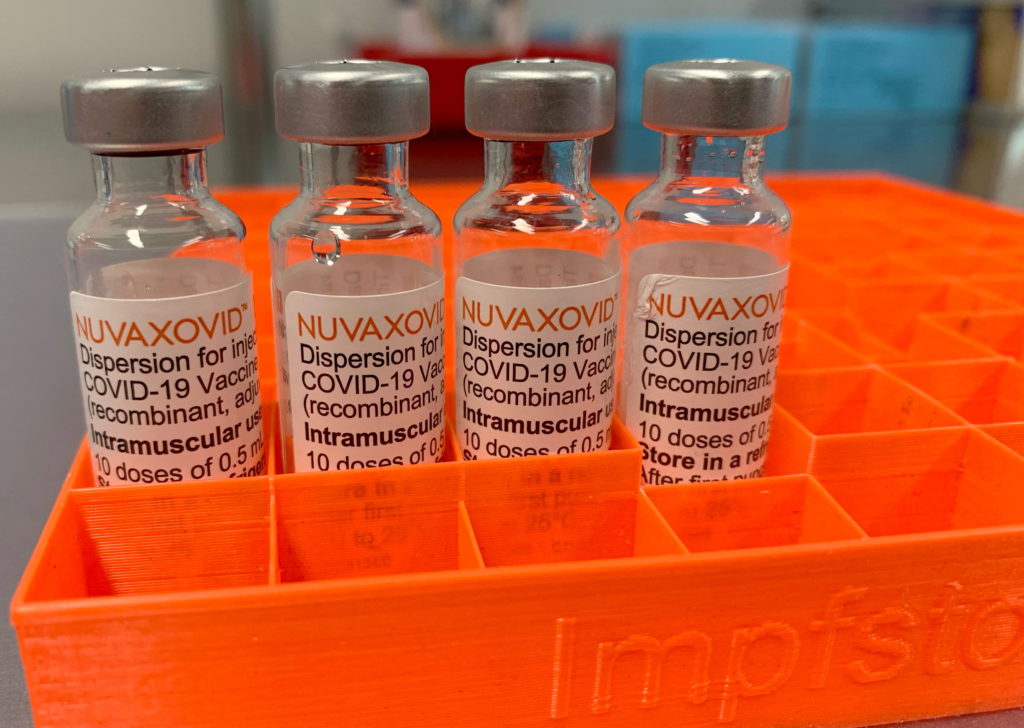
Novavax COVID-19 Vaccine Approved by FDA, but with Unusual Limitations
The FDA has finally granted approval to Novavax's COVID-19 vaccine, Nuvaxovid, but the announcement is tempered by unusual limitations that have raised eyebrows within the medical community. While this marks a significant milestone for Novavax and offers another option for those seeking COVID-19 protection, the restricted authorization highlights the complexities of vaccine approval and deployment in the evolving pandemic landscape.
A Long-Awaited Approval, but Why the Hesitation?
Novavax's vaccine, a protein subunit vaccine using a different technology than the mRNA vaccines from Pfizer-BioNTech and Moderna, has been eagerly anticipated. Its traditional approach has appealed to some individuals hesitant about mRNA technology. However, the FDA's approval comes significantly later than those of its competitors, and with caveats that limit its widespread use.
The FDA's approval is only for individuals 18 years of age and older. This is a noteworthy limitation, especially considering that other COVID-19 vaccines have received authorization for younger age groups. Furthermore, the agency's authorization is based on data showing the vaccine’s efficacy against the original strain of the virus. With the emergence of numerous variants, including Omicron and its subvariants, concerns about its effectiveness against currently circulating strains are paramount.
What are the Specific Limitations?
The FDA's authorization of Nuvaxovid is notably restricted compared to other authorized COVID-19 vaccines. Key limitations include:
- Limited Age Range: Approval is currently restricted to adults 18 years and older. Clinical trials for younger age groups are still ongoing.
- Efficacy Against Variants: The FDA's approval primarily reflects the vaccine's efficacy against the original COVID-19 strain. Its effectiveness against currently circulating variants remains a significant question. Further studies are needed to fully assess its protection against these evolving strains.
- Lower Demand: Given the extensive availability of other COVID-19 vaccines and the overall decline in COVID-19 cases, the demand for Novavax's vaccine might be limited, potentially impacting the vaccine's widespread distribution and utilization.
What Does this Mean for the Public?
The FDA's approval, albeit with limitations, expands the options available for individuals seeking COVID-19 vaccination. However, those considering Nuvaxovid should be aware of the restricted age range and the uncertainty surrounding its effectiveness against current variants. It's crucial to discuss the available options with a healthcare provider to determine the best vaccine for individual needs and risk factors.
Future Outlook and Ongoing Research:
Novavax is continuing its research and clinical trials to address the limitations. Further data on the vaccine's efficacy against emerging variants and its safety and effectiveness in younger age groups is expected. The FDA's ongoing monitoring will also be critical in evaluating the long-term impact and effectiveness of Nuvaxovid.
Conclusion:
The FDA's approval of the Novavax COVID-19 vaccine is a step forward, but the unusual limitations underscore the dynamic nature of vaccine development and deployment during a pandemic. While the vaccine offers an alternative for some, it's essential to understand its current restrictions and to consult a healthcare professional for personalized advice on COVID-19 vaccination. Staying informed about ongoing research and updates from the FDA is crucial in making informed decisions about protecting your health. For the latest updates on COVID-19 vaccines, consult the and your healthcare provider.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Approved By FDA With Unusual Limitations. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Lizzo Opens Up About The Impact Of Online Comments A Two Year Journey
May 20, 2025
Lizzo Opens Up About The Impact Of Online Comments A Two Year Journey
May 20, 2025 -
 Gaza Hospital Hit Israeli Strikes Target Last Northern Facility
May 20, 2025
Gaza Hospital Hit Israeli Strikes Target Last Northern Facility
May 20, 2025 -
 From Outback To Cell Block Matthew Radaljs China Prison Experience
May 20, 2025
From Outback To Cell Block Matthew Radaljs China Prison Experience
May 20, 2025 -
 The Last Of Us Exploring The Power Of Emotional Storytelling In Games
May 20, 2025
The Last Of Us Exploring The Power Of Emotional Storytelling In Games
May 20, 2025 -
 Investor Confidence In Ethereum Soars 200 M Investment Post Pectra
May 20, 2025
Investor Confidence In Ethereum Soars 200 M Investment Post Pectra
May 20, 2025
Latest Posts
-
 The Contradictory Reality Of Trumps Trade Policies One Factorys Story
May 20, 2025
The Contradictory Reality Of Trumps Trade Policies One Factorys Story
May 20, 2025 -
 Jon Jones Claims Ufc Withheld Crucial Aspinall Fight Information
May 20, 2025
Jon Jones Claims Ufc Withheld Crucial Aspinall Fight Information
May 20, 2025 -
 Eu Uk Brexit Talks Reach Critical Point A Deal Or A Disaster
May 20, 2025
Eu Uk Brexit Talks Reach Critical Point A Deal Or A Disaster
May 20, 2025 -
 Analysis Jon Jones Aspinall Comments And The Implications For The Ufc Heavyweight Title
May 20, 2025
Analysis Jon Jones Aspinall Comments And The Implications For The Ufc Heavyweight Title
May 20, 2025 -
 What Is Femicide Examining The Factors Contributing To Its Rise
May 20, 2025
What Is Femicide Examining The Factors Contributing To Its Rise
May 20, 2025
