Novavax COVID-19 Vaccine: FDA Approval And Key Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine: FDA Approval, Effectiveness, and Key Usage Restrictions
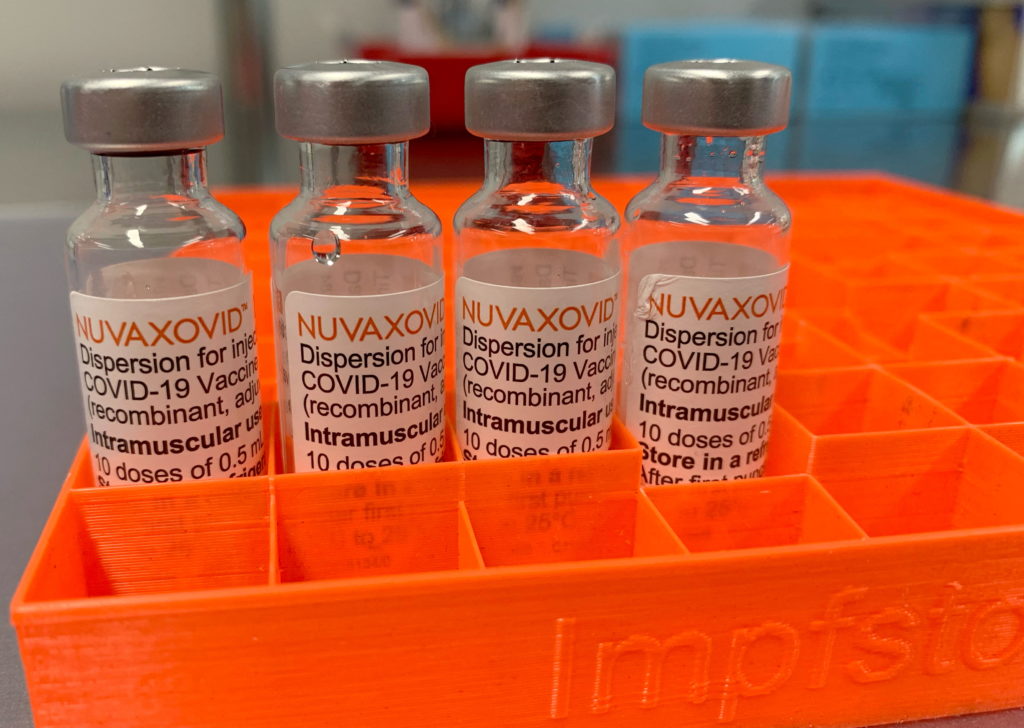
The Novavax COVID-19 vaccine, marketed as Nuvaxovid, represents a significant addition to the global fight against the pandemic. Its arrival offered a different approach to vaccination, leveraging protein subunit technology instead of mRNA, a factor that initially generated considerable public interest. However, its path to widespread adoption has been marked by complexities, including FDA approval timelines and specific usage restrictions. This article delves into the key details surrounding the vaccine's approval and limitations.
FDA Approval and Emergency Use Authorization (EUA):
The Novavax vaccine received Emergency Use Authorization (EUA) from the FDA in July 2022, followed by full FDA approval in July 2023 for individuals 18 years of age and older. This approval marked a crucial milestone, lending further credibility to the vaccine's safety and efficacy profile. The rigorous FDA review process ensured that the vaccine met stringent quality, safety, and effectiveness standards before gaining full authorization. This approval differs significantly from the EUA process, offering greater assurance to the public.
Mechanism of Action and Efficacy:
Unlike mRNA vaccines like Pfizer-BioNTech and Moderna, Novavax utilizes a protein subunit technology. This approach involves using harmless pieces of the virus's protein – the spike protein – to trigger an immune response. This method has been used successfully in other vaccines for years, offering a familiar approach for some individuals hesitant towards the newer mRNA technology. Clinical trials demonstrated strong efficacy against COVID-19, showing high rates of protection against severe illness, hospitalization, and death. While the initial efficacy rates might not have matched some mRNA vaccines in the early stages of the pandemic (variant-specific efficacy varies), the Novavax vaccine still plays a valuable role in preventing severe outcomes. Further research is continuously evaluating its effectiveness against emerging variants.
Key Usage Restrictions:
Despite its approval, certain restrictions and considerations surround the Novavax vaccine's usage:
- Age Restrictions: While approved for individuals 18 years and older, it's crucial to note that data on its use in younger populations is still limited. Its use in children and adolescents requires further study.
- Allergic Reactions: As with all vaccines, the possibility of allergic reactions exists. Individuals with a history of severe allergic reactions to any vaccine component should consult their healthcare provider before receiving the Novavax vaccine. Specific ingredients should be reviewed carefully.
- Storage and Handling: Maintaining the correct storage temperature is crucial for vaccine potency. Healthcare providers must adhere to strict guidelines to ensure the vaccine's effectiveness.
- Boosters: While initially approved as a primary vaccination series, the role of Novavax as a booster shot is still being investigated and may depend on individual circumstances and emerging variant data.
Conclusion:
The Novavax COVID-19 vaccine provides a valuable alternative for individuals seeking a non-mRNA vaccine option. Its FDA approval signifies a significant achievement in vaccine development and offers an additional tool in the global fight against COVID-19. However, it’s crucial to understand the specific usage restrictions and consult with a healthcare professional to determine the most appropriate vaccination strategy based on individual medical history and risk factors. Staying informed about the latest research and guidelines is essential in making informed decisions about COVID-19 vaccination. For the most up-to-date information, refer to the official websites of the FDA and the CDC.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein subunit vaccine, mRNA vaccine, vaccine efficacy, vaccine safety, usage restrictions, allergic reactions, booster shot, COVID-19 vaccination, pandemic, public health.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval And Key Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Wall Street Buoyancy Six Day Win Streak For S And P 500 Amidst Moodys Negative Outlook
May 21, 2025
Wall Street Buoyancy Six Day Win Streak For S And P 500 Amidst Moodys Negative Outlook
May 21, 2025 -
 Investor Confidence In Ethereum Soars 200 Million Investment Following Pectra
May 21, 2025
Investor Confidence In Ethereum Soars 200 Million Investment Following Pectra
May 21, 2025 -
 Tom Aspinall Negotiations Stall Jon Jones I M Done Tweet Sparks Retirement Rumors
May 21, 2025
Tom Aspinall Negotiations Stall Jon Jones I M Done Tweet Sparks Retirement Rumors
May 21, 2025 -
 Heated Debate Jon Jones Strip The Duck Comment On Tom Aspinall
May 21, 2025
Heated Debate Jon Jones Strip The Duck Comment On Tom Aspinall
May 21, 2025 -
 Down To The Wire Eu Uk Brexit Negotiations Face Impasse
May 21, 2025
Down To The Wire Eu Uk Brexit Negotiations Face Impasse
May 21, 2025
Latest Posts
-
 Autonomous Vehicles In The Uk Ubers 2023 Claim Vs Projected 2027 Deployment
May 21, 2025
Autonomous Vehicles In The Uk Ubers 2023 Claim Vs Projected 2027 Deployment
May 21, 2025 -
 Fda Approval Of Novavax Covid 19 Vaccine Understanding The Specific Restrictions
May 21, 2025
Fda Approval Of Novavax Covid 19 Vaccine Understanding The Specific Restrictions
May 21, 2025 -
 I M Done Jon Jones Hints At Ufc Retirement Amidst Aspinall Contract Dispute
May 21, 2025
I M Done Jon Jones Hints At Ufc Retirement Amidst Aspinall Contract Dispute
May 21, 2025 -
 Uk Driverless Cars 2027 Target Date Ubers Early Assertion
May 21, 2025
Uk Driverless Cars 2027 Target Date Ubers Early Assertion
May 21, 2025 -
 Gazas North Israeli Strikes Leave No Hospitals Operational
May 21, 2025
Gazas North Israeli Strikes Leave No Hospitals Operational
May 21, 2025
