Novavax COVID-19 Vaccine: FDA Approval And The Specifics Of Its Limited Use
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine: FDA Approval, Limited Use, and What You Need to Know
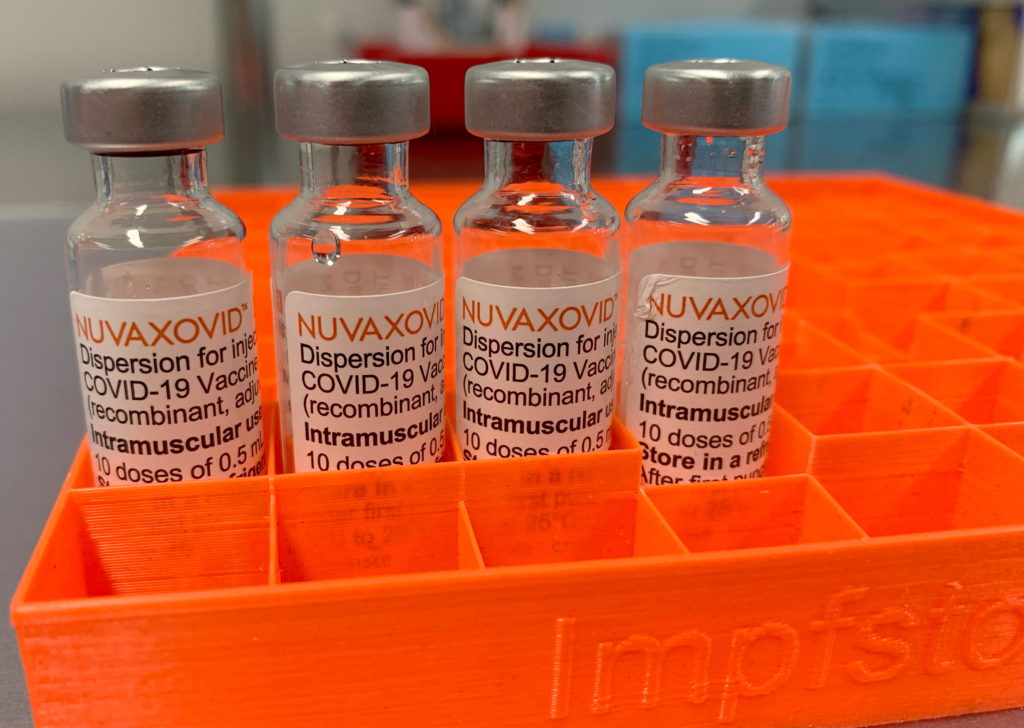
The Novavax COVID-19 vaccine, Nuvaxovid (also known as Covovax internationally), finally secured FDA approval in July 2023, marking a significant development in the fight against the pandemic. However, its journey to widespread use has been marked by a unique set of circumstances, resulting in a more limited application than other available vaccines. This article delves into the specifics of the FDA's approval, the reasons behind its restricted use, and what this means for individuals considering this vaccine option.
FDA Approval and Authorization:
The FDA's approval was granted under the agency's traditional licensure pathway, a more rigorous process than the Emergency Use Authorization (EUA) used for other COVID-19 vaccines initially. This approval signifies a thorough review of the vaccine's safety and efficacy data, bolstering confidence in its quality. However, it's crucial to note that while approved, its use remains more limited than other widely available vaccines.
Why the Limited Use?
Several factors contribute to Novavax's currently limited application:
- Late Entry into the Market: By the time Novavax received its approval, other vaccines had already established themselves, leading to lower demand. The initial surge in vaccination rates had subsided, and many individuals had already received alternative vaccines.
- Efficacy Data: While the vaccine demonstrated efficacy against COVID-19, its performance may not be as superior compared to other mRNA vaccines, particularly against newer variants. [Link to relevant clinical trial data].
- Logistics and Distribution: The manufacturing and distribution processes for Novavax might present unique challenges compared to more established vaccine manufacturers, impacting widespread availability.
- Safety Concerns: Although the FDA approval indicates a robust safety profile, post-market surveillance will continue to monitor for any rare adverse events. [Link to FDA safety information].
Who Should Consider the Novavax Vaccine?
Despite its limited use, the Novavax vaccine offers a valuable option for certain individuals:
- Individuals hesitant about mRNA vaccines: Novavax utilizes a different technology – a protein subunit vaccine – which may appeal to those with concerns about mRNA vaccines. This approach uses a piece of the virus, not the mRNA genetic code.
- Individuals seeking an alternative: Having another approved vaccine provides increased choice and may address supply chain issues or individual preferences.
- Specific populations undergoing clinical trials: ongoing trials might investigate the vaccine's efficacy and safety in specific populations.
Looking Ahead:
While Novavax's role in the broader COVID-19 vaccination landscape might be currently less prominent than others, its approval remains a significant achievement. Ongoing monitoring of its efficacy and safety, along with potential future adaptations to target emerging variants, will determine its long-term impact. The availability of the Novavax vaccine continues to be assessed by various health authorities globally, with future potential for wider adoption.
Call to Action:
Consult with your healthcare provider to determine which COVID-19 vaccine is best suited for your individual needs and circumstances. Staying informed about vaccine updates is crucial for making well-informed healthcare decisions. [Link to CDC website for updated vaccine information].

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval And The Specifics Of Its Limited Use. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Mlb Betting Predictions Walk Off Wagers On White Sox Vs Cubs And Red Sox Vs Braves
May 20, 2025
Mlb Betting Predictions Walk Off Wagers On White Sox Vs Cubs And Red Sox Vs Braves
May 20, 2025 -
 Investigation Reveals Lufthansa Flights 10 Minute Pilotless Operation After Co Pilots Medical Emergency
May 20, 2025
Investigation Reveals Lufthansa Flights 10 Minute Pilotless Operation After Co Pilots Medical Emergency
May 20, 2025 -
 Freaky Friday Reunion Jamie Lee Curtis Gives An Update On Her Friendship With Lindsay Lohan
May 20, 2025
Freaky Friday Reunion Jamie Lee Curtis Gives An Update On Her Friendship With Lindsay Lohan
May 20, 2025 -
 Peaky Blinders Creator Reveals New Series Details And Significant Shift
May 20, 2025
Peaky Blinders Creator Reveals New Series Details And Significant Shift
May 20, 2025 -
 Jamie Lee Curtis Discusses Her Close Friendship With Lindsay Lohan
May 20, 2025
Jamie Lee Curtis Discusses Her Close Friendship With Lindsay Lohan
May 20, 2025
Latest Posts
-
 The Last Of Us Success A Study In Narrative Tension
May 20, 2025
The Last Of Us Success A Study In Narrative Tension
May 20, 2025 -
 200 Million Ethereum Investment Boost Pectra Upgrade Fuels Growth
May 20, 2025
200 Million Ethereum Investment Boost Pectra Upgrade Fuels Growth
May 20, 2025 -
 Investigating A Prehistoric Puzzle The Case Of The Canadian Pachyrhinosaurus Die Off
May 20, 2025
Investigating A Prehistoric Puzzle The Case Of The Canadian Pachyrhinosaurus Die Off
May 20, 2025 -
 Cnn Reports Israeli Strikes Hit Final Hospital In North Gaza
May 20, 2025
Cnn Reports Israeli Strikes Hit Final Hospital In North Gaza
May 20, 2025 -
 Why The Last Of Us Deliberate Pace Makes It Truly Great
May 20, 2025
Why The Last Of Us Deliberate Pace Makes It Truly Great
May 20, 2025
