Novavax COVID-19 Vaccine: FDA Approval And Usage Limitations Explained
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
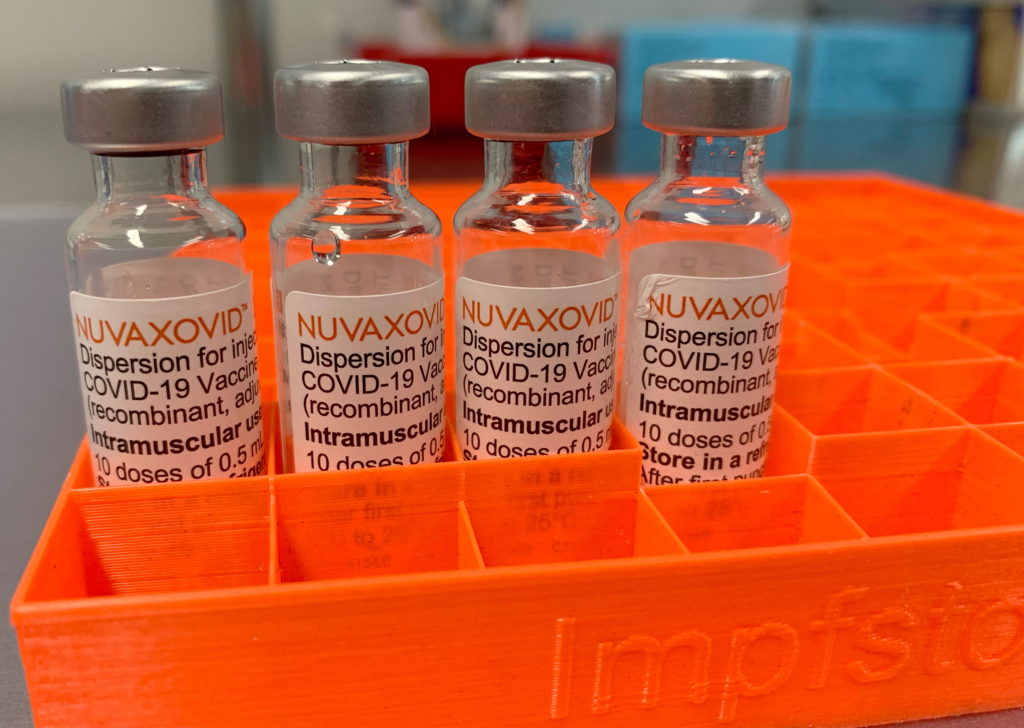
Novavax COVID-19 Vaccine: FDA Approval and Usage Limitations Explained
The arrival of the Novavax COVID-19 vaccine, Nuvaxovid, marked a significant development in the global fight against the pandemic. Offering a different approach compared to mRNA vaccines like Pfizer-BioNTech and Moderna, Novavax uses a more traditional protein subunit technology. However, its journey to widespread use has been marked by delays and specific limitations. This article will delve into the FDA approval process, efficacy, and the crucial usage limitations surrounding the Novavax vaccine.
FDA Approval and Emergency Use Authorization:
Unlike the rapid emergency use authorizations (EUAs) granted to other COVID-19 vaccines, Novavax received full FDA approval for individuals 18 years of age and older in July 2023. This followed an EUA granted earlier in the year for the same age group. This approval signifies a rigorous review process by the FDA, validating the vaccine's safety and efficacy. However, the relatively late arrival of the vaccine to the market means its use is now largely in a post-pandemic context, where other highly effective vaccines are already widely distributed and booster programs are established.
How Novavax Works: Protein Subunit Technology
Novavax's Nuvaxovid employs a protein subunit technology. This means it uses purified fragments of the virus's spike protein, rather than the mRNA code that instructs cells to produce the spike protein, as seen in mRNA vaccines. This traditional approach may appeal to individuals hesitant about mRNA technology. The protein subunits are combined with an adjuvant, a substance that enhances the immune response.
Efficacy and Safety:
Clinical trials demonstrated Novavax's efficacy in preventing COVID-19. While its efficacy rates might be slightly lower than some mRNA vaccines in initial trials, it still provides significant protection against severe illness, hospitalization, and death. The vaccine's safety profile, based on clinical trial data, is generally considered comparable to other approved COVID-19 vaccines. However, as with all vaccines, side effects like pain at the injection site, fatigue, and headache are possible.
Usage Limitations: Why isn't everyone using it?
Despite FDA approval, the Novavax vaccine's usage is currently limited by several factors:
- Lower Demand: With other readily available and highly effective vaccines, demand for Novavax has been comparatively low. This has impacted its widespread distribution and accessibility.
- Production Challenges: Production issues initially hampered the vaccine's rollout. These logistical challenges contributed to its later arrival on the market.
- Specific Age Groups: While approved for adults 18 years and older, the FDA has not yet approved Novavax for younger age groups. Further trials are likely needed before such approval is granted.
- Booster Strategy: The role of Novavax in booster strategies remains unclear and is still under investigation.
Conclusion:
The Novavax COVID-19 vaccine offers a viable alternative for those who prefer a protein subunit vaccine over mRNA technology. While it received full FDA approval, its impact on the current landscape of COVID-19 vaccination is limited by lower demand, past production challenges, and ongoing research regarding its usage in specific populations and booster scenarios. Individuals considering the Novavax vaccine should consult with their healthcare provider to discuss its suitability based on their individual health circumstances and risk factors. For the latest information on COVID-19 vaccines, refer to the and .
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein subunit vaccine, vaccine efficacy, vaccine safety, usage limitations, mRNA vaccines, COVID-19 vaccination, vaccine rollout, booster shot, COVID-19 pandemic.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval And Usage Limitations Explained. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Animal Rescue Emergency Delaware Shelter Struggles To Care For Abandoned Chicks From Usps Truck
May 21, 2025
Animal Rescue Emergency Delaware Shelter Struggles To Care For Abandoned Chicks From Usps Truck
May 21, 2025 -
 Helping Your Child Give Up Their Pacifier Or Thumb Expert Advice For Parents
May 21, 2025
Helping Your Child Give Up Their Pacifier Or Thumb Expert Advice For Parents
May 21, 2025 -
 Jamie Lee Curtis Discusses Her Connection With Lindsay Lohan A Candid Interview
May 21, 2025
Jamie Lee Curtis Discusses Her Connection With Lindsay Lohan A Candid Interview
May 21, 2025 -
 Federal Assault Charges Filed Against Congresswoman Newark Mayor Case Dropped
May 21, 2025
Federal Assault Charges Filed Against Congresswoman Newark Mayor Case Dropped
May 21, 2025 -
 Nick Ut And The Napalm Girl World Press Photo Re Examines Iconic Image
May 21, 2025
Nick Ut And The Napalm Girl World Press Photo Re Examines Iconic Image
May 21, 2025
Latest Posts
-
 Police Investigate Church Break In Report Of Defecation
May 21, 2025
Police Investigate Church Break In Report Of Defecation
May 21, 2025 -
 Trump Doj And James A Complex Web Of Legal Battles
May 21, 2025
Trump Doj And James A Complex Web Of Legal Battles
May 21, 2025 -
 Diplomatic Tensions Rise As Allies Demand End To Israels Gaza Operation
May 21, 2025
Diplomatic Tensions Rise As Allies Demand End To Israels Gaza Operation
May 21, 2025 -
 New Orleans Jailbreak Fourth Inmate Captured As District Attorneys Staff Seek Safety
May 21, 2025
New Orleans Jailbreak Fourth Inmate Captured As District Attorneys Staff Seek Safety
May 21, 2025 -
 Police Teenagers Arrested For Urinating And Defecating In Santa Rosa Church
May 21, 2025
Police Teenagers Arrested For Urinating And Defecating In Santa Rosa Church
May 21, 2025
