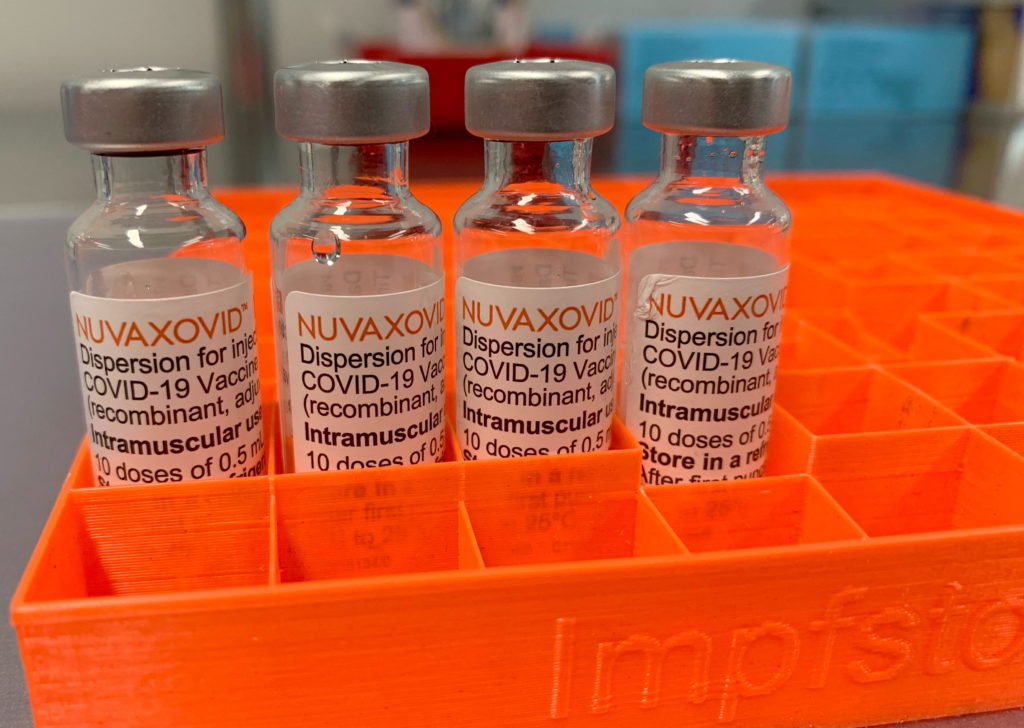
Novavax COVID-19 Vaccine: FDA Approval Comes With Specific Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine: FDA Approval Comes With Specific Usage Restrictions
The Novavax COVID-19 vaccine, Nuvaxovid, has finally received full FDA approval, marking a significant milestone in the fight against the pandemic. However, this approval comes with important caveats: specific usage restrictions that limit its immediate widespread application. This news offers both good and bad news for those eagerly awaiting a protein-subunit vaccine option. Let's delve into the details.
What's the Big Deal About FDA Approval?
Full FDA approval signifies a rigorous review process exceeding the emergency use authorization (EUA) under which many COVID-19 vaccines were previously administered. This means the FDA has conducted extensive analyses of the vaccine's safety and efficacy data, providing a higher level of confidence in its performance. This could potentially increase vaccine uptake among hesitant individuals who prefer fully approved options.
The Nuvaxovid Nuances: Usage Restrictions Explained
While approval is welcome news, the FDA's authorization isn't blanket approval for all individuals. The approval is specifically for individuals 18 years of age and older. This excludes adolescents and children, a key demographic still vulnerable to COVID-19. Further research is needed to assess the vaccine's efficacy and safety in younger populations.
The FDA also emphasizes that Nuvaxovid should be administered as a two-dose primary series, with doses given at least three weeks apart. This aligns with the standard regimen for many other COVID-19 vaccines. However, information regarding booster doses and their necessity for long-term protection remains under review and will likely be updated by the FDA in the coming months.
Why the Specific Restrictions?
The FDA's decision to impose restrictions likely stems from a cautious approach to ensuring public safety. While the efficacy data is promising, further trials are necessary to fully understand the long-term effects and efficacy in all age groups. This also allows for continuous monitoring of post-market safety data and potential adverse effects.
What Does This Mean for the Future of COVID-19 Vaccination?
The Novavax vaccine offers a different approach than mRNA vaccines like Pfizer-BioNTech and Moderna, utilizing a protein-subunit technology. This might appeal to individuals hesitant about mRNA vaccines due to concerns about the technology. This diversification of vaccine types is crucial in tackling the pandemic, providing options tailored to individual preferences and medical needs.
However, the restricted approval means that Novavax's immediate impact on the overall vaccination landscape might be less dramatic than initially hoped. The focus will likely remain on existing authorized vaccines for broader application, especially in pediatric populations.
Looking Ahead: Next Steps and Further Research
Further clinical trials are likely underway to expand the authorized usage of Nuvaxovid to include adolescents and children. Additional research will also focus on long-term efficacy and the need for booster shots. The FDA will continuously monitor safety data to ensure the vaccine's ongoing safety and effectiveness.
Conclusion:
The FDA's approval of the Novavax COVID-19 vaccine is undoubtedly positive news, introducing a new protein-subunit vaccine into the mix. However, the associated usage restrictions highlight the ongoing need for further research and caution in deploying novel vaccines. This measured approach underscores the FDA's commitment to prioritizing public safety and ensuring the efficacy of all authorized vaccines. Stay informed about further updates from the FDA and your healthcare provider for the most current information on COVID-19 vaccination.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein-subunit vaccine, vaccine safety, vaccine efficacy, usage restrictions, vaccination, COVID-19, pandemic, public health.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval Comes With Specific Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Immediate Russia Ukraine Truce Talks Trumps Latest Statement
May 20, 2025
Immediate Russia Ukraine Truce Talks Trumps Latest Statement
May 20, 2025 -
 Watch Now Critically Acclaimed Wwi Movie With All Star Cast
May 20, 2025
Watch Now Critically Acclaimed Wwi Movie With All Star Cast
May 20, 2025 -
 Understanding Femicide A Deep Dive Into The Global Crisis
May 20, 2025
Understanding Femicide A Deep Dive Into The Global Crisis
May 20, 2025 -
 Understanding The New Buy Now Pay Later Regulations What Consumers Need To Know
May 20, 2025
Understanding The New Buy Now Pay Later Regulations What Consumers Need To Know
May 20, 2025 -
 Clues From The Past A Canadian Pachyrhinosaurus Mass Grave
May 20, 2025
Clues From The Past A Canadian Pachyrhinosaurus Mass Grave
May 20, 2025
Latest Posts
-
 Bali Urges Global Cooperation For Responsible Tourism Practices
May 20, 2025
Bali Urges Global Cooperation For Responsible Tourism Practices
May 20, 2025 -
 Bali Tourism Overhaul New Regulations Target Bad Tourist Conduct
May 20, 2025
Bali Tourism Overhaul New Regulations Target Bad Tourist Conduct
May 20, 2025 -
 Watch A Powerful Wwi Story Starring Daniel Craig Cillian Murphy And Tom Hardy Streaming Now
May 20, 2025
Watch A Powerful Wwi Story Starring Daniel Craig Cillian Murphy And Tom Hardy Streaming Now
May 20, 2025 -
 Weaning Your Child Off Pacifiers And Thumbs Age Tips And Advice
May 20, 2025
Weaning Your Child Off Pacifiers And Thumbs Age Tips And Advice
May 20, 2025 -
 Canadian Fossil Discovery New Insights Into Pachyrhinosaurus Death
May 20, 2025
Canadian Fossil Discovery New Insights Into Pachyrhinosaurus Death
May 20, 2025
