Novavax COVID-19 Vaccine: FDA Approval Comes With Strict Conditions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine: FDA Approval Comes With Strict Conditions
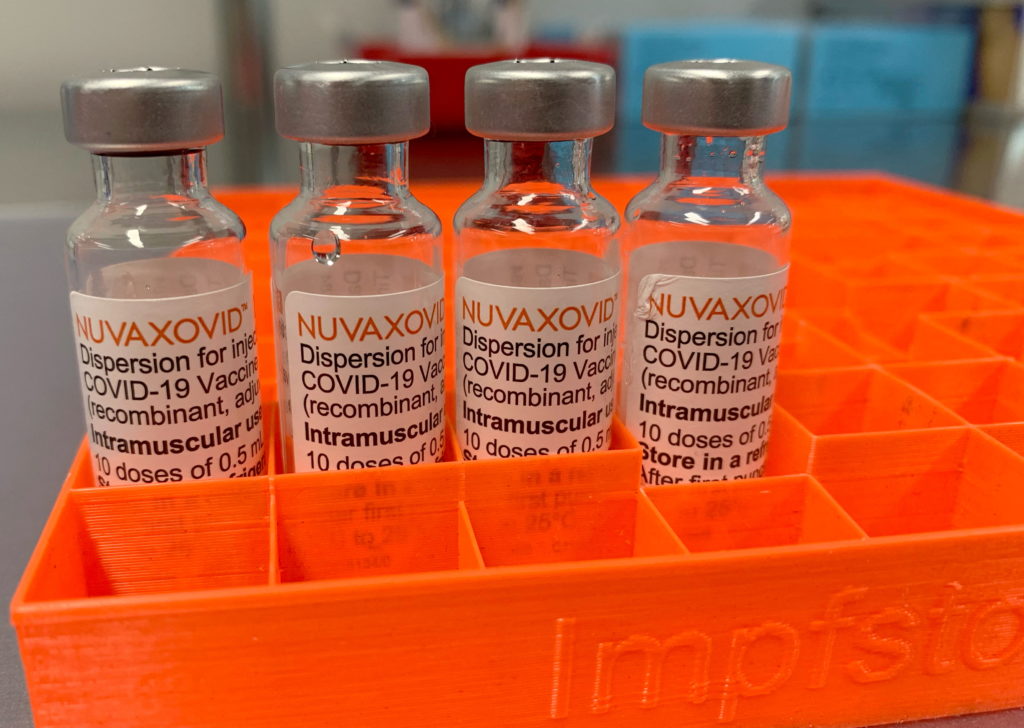
The Novavax COVID-19 vaccine, Nuvaxovid, finally received FDA approval in July 2023, but the green light comes with significant caveats. This marks a significant development in the fight against the pandemic, offering an alternative option for those hesitant about mRNA vaccines. However, the stringent conditions imposed by the FDA raise important questions about its long-term role in vaccination strategies.
A Long-Awaited Approval, But With Strings Attached
The FDA's approval wasn't a straightforward rubber stamp. The agency's decision was heavily influenced by ongoing safety concerns and a relatively lower efficacy rate compared to other authorized vaccines. While Nuvaxovid demonstrated efficacy against COVID-19, particularly against severe illness and hospitalization, the data presented wasn't as compelling as that for Pfizer-BioNTech or Moderna vaccines. This contributed to the imposition of strict conditions on its use.
What are the Conditions of FDA Approval?
The FDA's approval is contingent upon several factors, including:
- Post-Market Surveillance: Novavax is required to conduct extensive post-market surveillance to continuously monitor the vaccine's safety and effectiveness in the real world. This includes tracking adverse events and evaluating its efficacy against emerging variants.
- Manufacturing Oversight: The FDA will maintain strict oversight of the vaccine's manufacturing process to ensure consistent quality and safety. This is crucial given concerns about potential production issues that previously delayed the vaccine's rollout.
- Data Reporting: Novavax is obligated to provide regular updates to the FDA on various data points, including adverse event reports, manufacturing details, and efficacy data against new variants. This stringent reporting requirement ensures ongoing transparency and accountability.
Why the Strict Conditions?
The FDA's cautious approach is likely due to several factors:
- Comparative Efficacy: As mentioned earlier, Nuvaxovid's efficacy, while still significant, was comparatively lower than other authorized vaccines in clinical trials.
- Safety Concerns: Although generally well-tolerated, some safety concerns emerged during clinical trials, prompting further scrutiny from the FDA.
- Manufacturing Challenges: Early reports of manufacturing challenges raised concerns about the vaccine's consistent quality and availability.
The Future of Novavax in the COVID-19 Vaccination Landscape
Despite the strict conditions, the FDA approval of the Novavax vaccine represents a valuable addition to the available COVID-19 vaccines. It provides an alternative for individuals who prefer a protein-based vaccine over mRNA technology. However, the long-term impact of the vaccine's use will depend heavily on the results of ongoing post-market surveillance and the ongoing evolution of the virus. The efficacy against new variants will be a key factor in determining its continued relevance.
What does this mean for you?
The approval of the Novavax vaccine broadens the options available for COVID-19 vaccination. Consult your physician to determine the most suitable vaccine for your individual needs and circumstances. While this offers a choice, remember that all authorized COVID-19 vaccines are effective in reducing the risk of severe illness and hospitalization. Staying updated on the latest guidelines from the CDC and your healthcare provider remains crucial in managing your risk of COVID-19 infection.
Further Reading:
This article is for informational purposes only and does not constitute medical advice. Always consult with a healthcare professional before making any decisions related to your health or treatment.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval Comes With Strict Conditions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Impact Of Feds 2025 Rate Cut Projection On Us Treasury Yields
May 21, 2025
Impact Of Feds 2025 Rate Cut Projection On Us Treasury Yields
May 21, 2025 -
 Jamie Lee Curtis And Lindsay Lohan A Real Friendship Revealed
May 21, 2025
Jamie Lee Curtis And Lindsay Lohan A Real Friendship Revealed
May 21, 2025 -
 Usps Shipment Disaster Delaware Shelter Struggles With Thousands Of Neglected Chicks
May 21, 2025
Usps Shipment Disaster Delaware Shelter Struggles With Thousands Of Neglected Chicks
May 21, 2025 -
 Jon Jones Vs Tom Aspinall The Controversy Behind The Strip The Duck Remark
May 21, 2025
Jon Jones Vs Tom Aspinall The Controversy Behind The Strip The Duck Remark
May 21, 2025 -
 Trumps Call For Immediate Russia Ukraine Peace Negotiations
May 21, 2025
Trumps Call For Immediate Russia Ukraine Peace Negotiations
May 21, 2025
Latest Posts
-
 Faa And Ntsb Investigate Close Call At La Guardia Airport
May 21, 2025
Faa And Ntsb Investigate Close Call At La Guardia Airport
May 21, 2025 -
 Ubisofts Design Choice Why Animals Are Untouchable In Assassins Creed Valhalla
May 21, 2025
Ubisofts Design Choice Why Animals Are Untouchable In Assassins Creed Valhalla
May 21, 2025 -
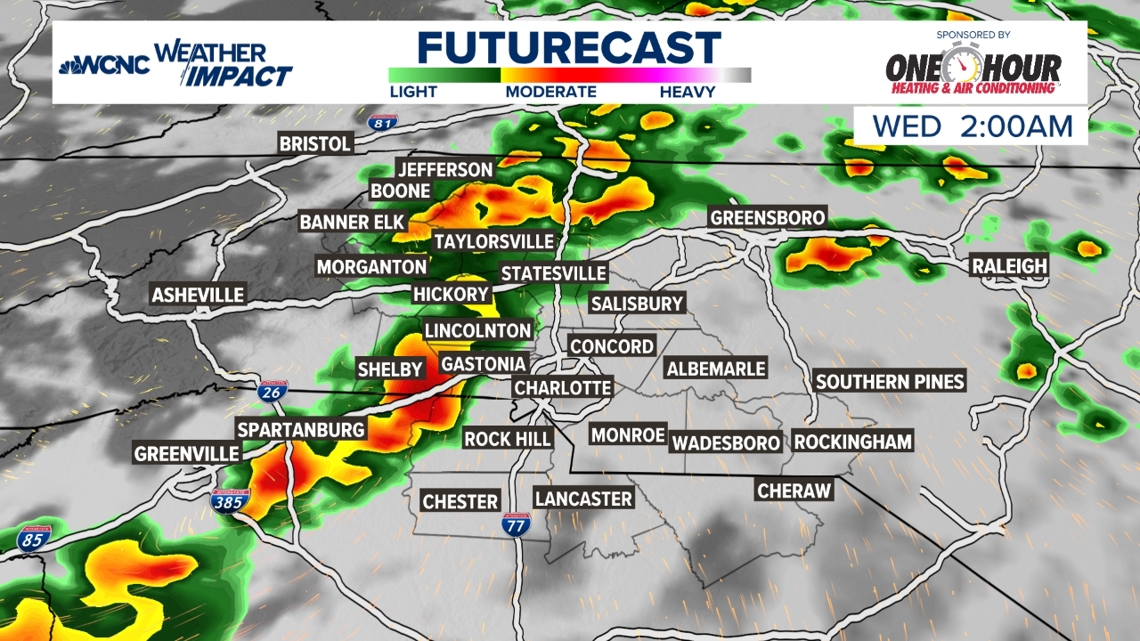 Weather Alert Small Risk Of Intense Storms Late Tuesday
May 21, 2025
Weather Alert Small Risk Of Intense Storms Late Tuesday
May 21, 2025 -
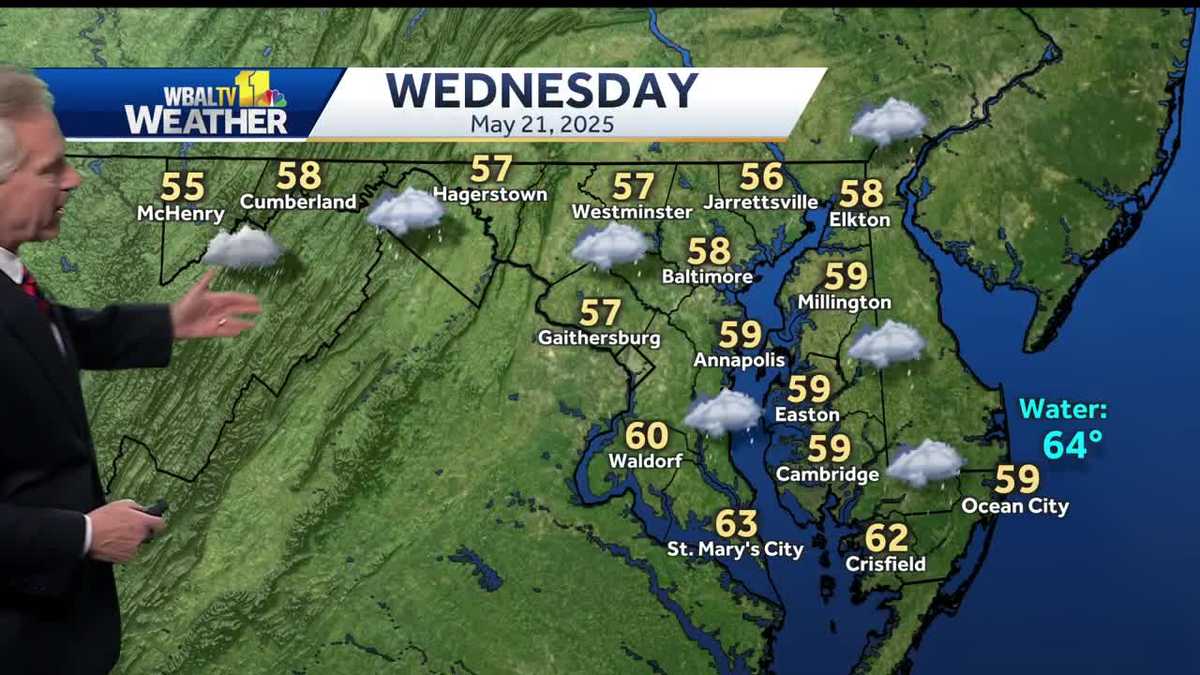 Widespread Rain And Cold To Hit Region Wednesday
May 21, 2025
Widespread Rain And Cold To Hit Region Wednesday
May 21, 2025 -
 Strange Case Of Baby Abductions By Monkeys On A Panamanian Island
May 21, 2025
Strange Case Of Baby Abductions By Monkeys On A Panamanian Island
May 21, 2025
