Novavax COVID-19 Vaccine: FDA Approval Comes With Unusual Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine: FDA Approval Comes With Unusual Restrictions
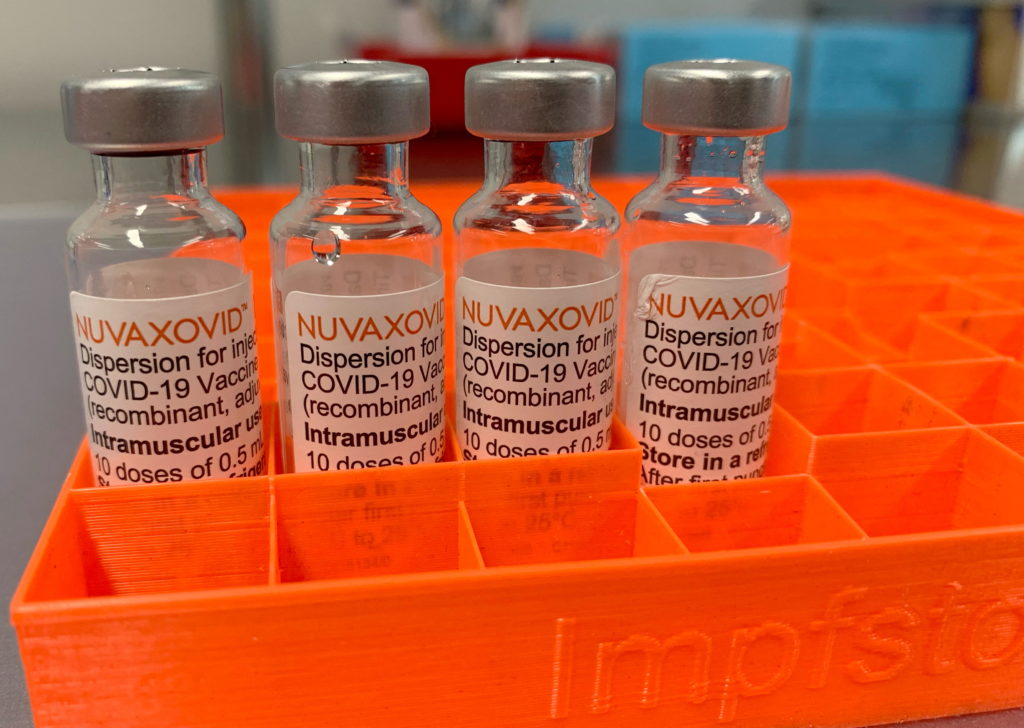
The Novavax COVID-19 vaccine, Nuvaxovid, finally received FDA approval in July 2023, marking a significant milestone in the fight against the pandemic. However, this approval comes with some unusual and noteworthy restrictions, raising questions about its future role in the vaccination landscape. Unlike the previously authorized mRNA vaccines and the Johnson & Johnson vaccine, Nuvaxovid's path to market has been marked by delays and now, a conditional approval with caveats.
This article delves into the specifics of these restrictions, exploring their implications for both public health and the pharmaceutical industry. We'll examine the reasons behind these limitations and discuss their potential impact on vaccine uptake and distribution.
Limited Authorization: Why the Conditional Approval?
The FDA granted Nuvaxovid Emergency Use Authorization (EUA) previously, but full approval came with specific conditions. While the vaccine's efficacy and safety profile have been established, the FDA's decision to attach these conditions highlights several factors:
- Lower Efficacy Compared to mRNA Vaccines: Clinical trials showed Nuvaxovid's efficacy to be lower compared to the Pfizer-BioNTech and Moderna vaccines, particularly against emerging variants. This difference in efficacy is a key factor in the conditional approval.
- Limited Data on Long-Term Effects: While the short-term safety and efficacy data is promising, the long-term effects of the Novavax vaccine are still under investigation. The FDA requires continued monitoring and data collection to fully assess its long-term safety profile.
- Manufacturing and Supply Chain Considerations: The approval also hinges on Novavax meeting stringent manufacturing and quality control standards. Any disruptions in the supply chain could affect the vaccine's availability.
What are the Practical Implications of these Restrictions?
These limitations translate into several practical challenges:
- Reduced Demand: The lower efficacy compared to other authorized vaccines might lead to reduced demand among individuals who have already received or are considering other COVID-19 vaccines.
- Targeted Distribution: The FDA might recommend prioritizing the Novavax vaccine for specific populations, such as those with concerns about mRNA vaccines, although this is yet to be officially outlined in detail.
- Ongoing Monitoring and Reporting: Novavax is required to conduct post-market surveillance and submit regular safety and efficacy data to the FDA, necessitating ongoing monitoring of its performance in real-world settings.
The Future of the Novavax Vaccine
The future role of the Novavax vaccine remains uncertain. While its approval provides an alternative for those hesitant about mRNA technology, the conditional nature of the approval and the lower reported efficacy compared to other options pose challenges. The vaccine's success will depend largely on ongoing monitoring, continued demonstration of safety and efficacy against new variants, and successful manufacturing and distribution. Furthermore, the evolving COVID-19 landscape, including the emergence of new variants and waning immunity, will significantly influence the vaccine's ongoing relevance.
Further Research and Resources: For more detailed information, consult the official FDA website and the CDC's website for the most up-to-date guidance on COVID-19 vaccines. Staying informed about the latest research and recommendations is crucial in making informed decisions about your health.
Disclaimer: This article provides general information and does not constitute medical advice. Always consult with a healthcare professional for any health concerns or before making any decisions related to your health or treatment.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval Comes With Unusual Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Eurovision 2025 Results Austria Takes The Crown Uk Falls Short
May 20, 2025
Eurovision 2025 Results Austria Takes The Crown Uk Falls Short
May 20, 2025 -
 Two Adults Dead Children Injured In Train Family Collision On Bridge
May 20, 2025
Two Adults Dead Children Injured In Train Family Collision On Bridge
May 20, 2025 -
 Jenn Stergers Emotional Account Of The Brett Favre Sexting Scandal Aftermath
May 20, 2025
Jenn Stergers Emotional Account Of The Brett Favre Sexting Scandal Aftermath
May 20, 2025 -
 Critically Acclaimed Ww 1 Film With Daniel Craig Cillian Murphy And Tom Hardy Available Now
May 20, 2025
Critically Acclaimed Ww 1 Film With Daniel Craig Cillian Murphy And Tom Hardy Available Now
May 20, 2025 -
 Weaning Your Child From A Pacifier Or Thumb Age Tips And Challenges
May 20, 2025
Weaning Your Child From A Pacifier Or Thumb Age Tips And Challenges
May 20, 2025
Latest Posts
-
 Colder Weather Arrives Expect Rain Through The Week
May 21, 2025
Colder Weather Arrives Expect Rain Through The Week
May 21, 2025 -
 Federal Inquiry Into New York Attorney General Fbi Directors Statement
May 21, 2025
Federal Inquiry Into New York Attorney General Fbi Directors Statement
May 21, 2025 -
 Familys Bitter Protest Law Change Threatens Paedophiles Parental Rights
May 21, 2025
Familys Bitter Protest Law Change Threatens Paedophiles Parental Rights
May 21, 2025 -
 The Eu Deal And The Uk Chris Mason Explains The Ongoing Challenges
May 21, 2025
The Eu Deal And The Uk Chris Mason Explains The Ongoing Challenges
May 21, 2025 -
 Charges Filed Against Second Suspect In Pms Home Fire Case
May 21, 2025
Charges Filed Against Second Suspect In Pms Home Fire Case
May 21, 2025
