Novavax COVID-19 Vaccine: FDA Grants Approval, But With Key Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine: FDA Grants Approval, But with Key Restrictions
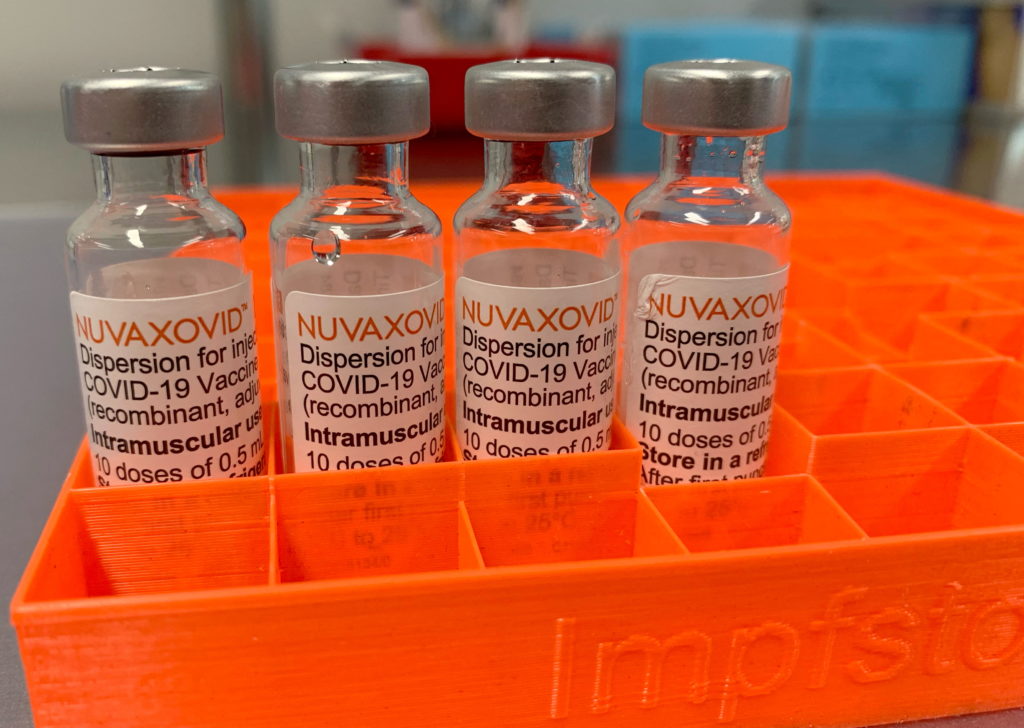
The FDA has finally granted approval to the Novavax COVID-19 vaccine, Nuvaxovid, offering a new option for those seeking protection against the virus. However, this approval comes with significant limitations, raising questions about its widespread adoption and impact on the ongoing pandemic. This article delves into the details of the approval, the restrictions imposed, and what this means for individuals and public health efforts.
A Long-Awaited Approval, but with Caveats
The approval of Nuvaxovid marks a significant milestone in the fight against COVID-19. Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, or the viral vector vaccine from Johnson & Johnson, Novavax utilizes a more traditional protein subunit technology. This protein-based approach has been touted by some as potentially appealing to vaccine-hesitant individuals who may have concerns about mRNA technology. However, the FDA's approval wasn't unconditional.
Key Restrictions Imposed by the FDA
The FDA's authorization is limited to individuals 18 years of age and older. This is a key restriction, as other COVID-19 vaccines have been authorized for younger age groups. Furthermore, the approval is specifically for a two-dose primary vaccination series, with no mention of booster shots at this time. The agency cited a need for further data on booster efficacy and safety before expanding the authorization.
This limited approval also impacts the vaccine's potential role in stemming the ongoing pandemic. With other vaccines already widely available and booster campaigns underway, the specific niche for Nuvaxovid remains unclear. The FDA's careful approach highlights a commitment to rigorous safety standards, but also raises questions about the vaccine's practical impact given its late entry into the market.
Why the Restrictions? Examining the FDA's Rationale
The FDA's decision to approve Nuvaxovid with these restrictions is likely driven by several factors. Firstly, the data submitted by Novavax for review may not have been as comprehensive as that submitted for other vaccines, particularly regarding long-term efficacy and safety profiles across different age groups. Secondly, the current landscape of COVID-19 vaccination is significantly different from when initial vaccine approvals were granted. With high vaccination rates in many countries and the emergence of new variants, the perceived urgency for a new vaccine may be lower.
What Does This Mean for the Future of COVID-19 Vaccination?
The approval of the Novavax vaccine, while positive news, is unlikely to dramatically alter the course of the pandemic in the short term. Its limited authorization, coupled with the existing availability of other vaccines, suggests a niche role for Nuvaxovid, potentially targeting specific populations or individuals who prefer this type of vaccine technology.
Further research and data are crucial to fully understand the long-term efficacy and safety of the Nuvaxovid vaccine, as well as its potential use as a booster. Only time will tell whether this vaccine will play a significant role in the ongoing efforts to control and prevent COVID-19 infections.
Looking Ahead: Future Prospects for Novavax and COVID-19 Vaccination
The next steps for Novavax involve gathering further data to support broader authorization, potentially including use in younger age groups and as a booster shot. The company's ability to efficiently conduct these studies and convince regulators of the vaccine's continued value will be key to determining its long-term success.
The approval of the Novavax vaccine underscores the complexities of vaccine development, approval, and deployment during a public health crisis. While offering a new choice, its restricted approval highlights the rigorous standards required for widespread adoption and the ongoing need for continued monitoring and research in the fight against COVID-19.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein subunit vaccine, vaccine restrictions, mRNA vaccine, vaccine hesitancy, public health, pandemic, COVID-19 vaccination, booster shot, vaccine efficacy, vaccine safety.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Grants Approval, But With Key Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Intolerable Suffering In Gaza And Brexits Reset A Global Update
May 21, 2025
Intolerable Suffering In Gaza And Brexits Reset A Global Update
May 21, 2025 -
 Police Investigate Desecration Of Santa Rosa Church Teenagers Involved
May 21, 2025
Police Investigate Desecration Of Santa Rosa Church Teenagers Involved
May 21, 2025 -
 Putins Snub A Public Display Of Trumps Diminished Influence
May 21, 2025
Putins Snub A Public Display Of Trumps Diminished Influence
May 21, 2025 -
 Stock Market Soars Six Day Winning Streak For S And P 500 Amidst Moodys Action
May 21, 2025
Stock Market Soars Six Day Winning Streak For S And P 500 Amidst Moodys Action
May 21, 2025 -
 Police Investigate Church Break In Two Boys Suspected
May 21, 2025
Police Investigate Church Break In Two Boys Suspected
May 21, 2025
Latest Posts
-
 Choosing Safe Sunscreen For 2025 A Family Guide
May 21, 2025
Choosing Safe Sunscreen For 2025 A Family Guide
May 21, 2025 -
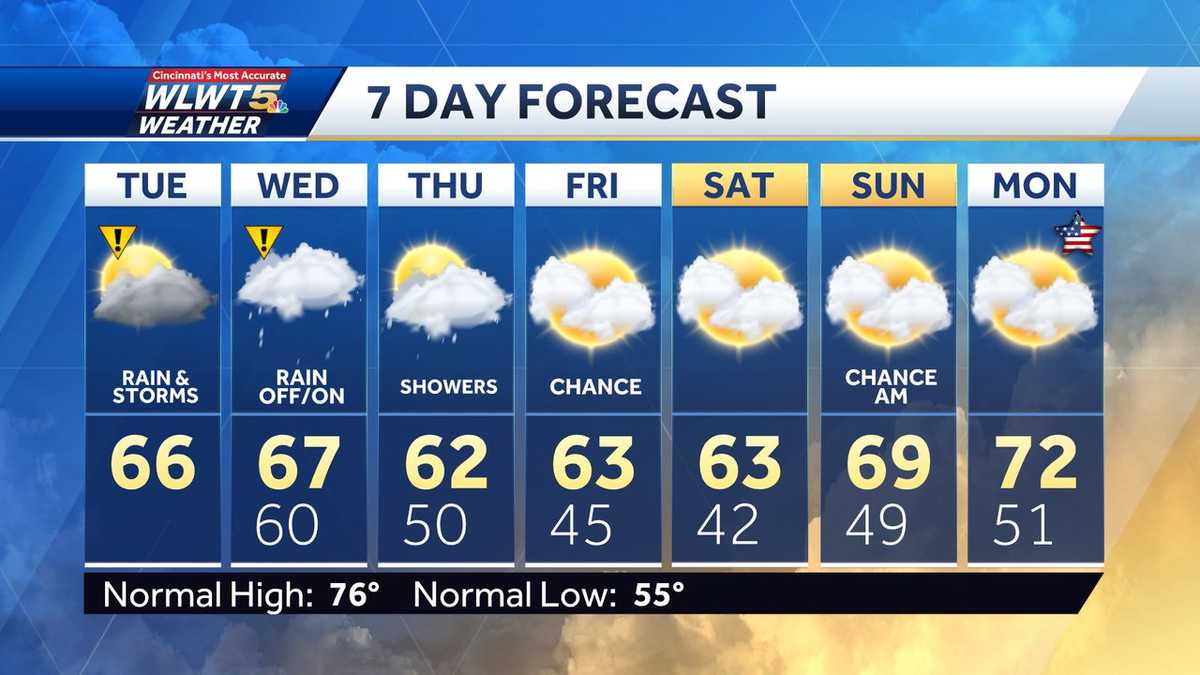 Cooler Weather Arrives Rain Likely Through The Week
May 21, 2025
Cooler Weather Arrives Rain Likely Through The Week
May 21, 2025 -
 Sesame Street From Trump Funding Cuts To Netflix Streaming Success
May 21, 2025
Sesame Street From Trump Funding Cuts To Netflix Streaming Success
May 21, 2025 -
 Analyzing The Impact Of Brexit And The Gaza Crisis A Global Perspective
May 21, 2025
Analyzing The Impact Of Brexit And The Gaza Crisis A Global Perspective
May 21, 2025 -
 Monkey Kidnappings On A Panamanian Island Unraveling The Strange Behavior
May 21, 2025
Monkey Kidnappings On A Panamanian Island Unraveling The Strange Behavior
May 21, 2025
