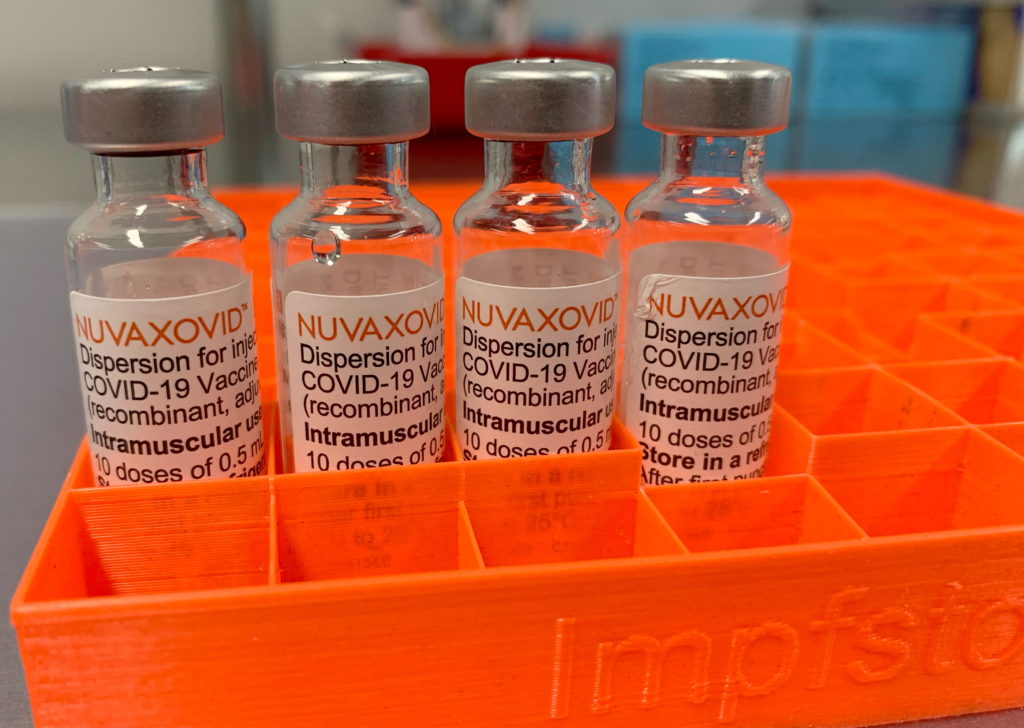
Novavax COVID-19 Vaccine Gets FDA Nod, But Under Specific Usage Guidelines
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Gets FDA Nod, but Under Specific Usage Guidelines
The FDA has finally granted Emergency Use Authorization (EUA) to the Novavax COVID-19 vaccine, Nuvaxovid, offering a new option in the fight against the pandemic. However, the authorization comes with specific usage guidelines, limiting its immediate impact. This development marks a significant milestone for Novavax, but also raises questions about the vaccine's role in the current landscape of readily available COVID-19 immunizations.
The approval, announced [Date of Announcement], grants EUA for individuals 18 years of age and older. This is a crucial distinction, as other widely available vaccines already boast authorization for broader age groups, including adolescents and children. The FDA's decision highlights the rigorous process involved in vaccine approval and underscores the agency's commitment to ensuring both efficacy and safety.
Why the Specific Usage Guidelines?
The FDA's decision to grant EUA under specific guidelines is multifaceted. While the vaccine demonstrated efficacy in clinical trials, its performance compared to already established mRNA vaccines like Pfizer-BioNTech and Moderna may have played a role. Furthermore, the evolving nature of the pandemic, with the emergence of new variants and waning immunity, necessitates a careful evaluation of new vaccine candidates.
The limited rollout might also be attributed to several factors including:
- Lower demand: With readily available and highly effective mRNA vaccines and other options already in widespread use, the demand for a new vaccine might be comparatively lower.
- Production capacity: Novavax's production capacity may currently limit the vaccine's immediate reach. Scaling up production to meet potential demand will be crucial for a wider rollout.
- Ongoing monitoring: The FDA will likely continue to monitor the vaccine's safety and efficacy in real-world settings, gathering further data before considering broader authorization.
What this Means for Individuals
For adults who prefer a protein-based vaccine over mRNA vaccines, the FDA's approval presents a new alternative. Those hesitant about mRNA technology may find this option more appealing. However, it's vital to understand that the FDA’s specific usage guidelines imply this vaccine won't be a primary choice for most.
Individuals considering the Novavax vaccine should consult with their healthcare provider. They can discuss the vaccine's potential benefits and risks in the context of individual health needs and existing immunity.
The Future of the Novavax Vaccine
The long-term prospects of the Novavax vaccine remain uncertain. Its success will depend on several factors, including public perception, manufacturing capacity, and the continued evolution of the COVID-19 virus. While it offers a different technological approach to vaccination, its role in the ongoing pandemic response remains to be seen. The FDA's continued monitoring and potential future expansion of the EUA will be key indicators of the vaccine's long-term impact.
This new development in the fight against COVID-19 underscores the importance of ongoing research and development in the face of evolving viral threats. Staying informed through reputable sources like the CDC and FDA websites is crucial for making informed decisions regarding vaccination. For more information on COVID-19 vaccines and vaccination strategies, you can visit the [link to CDC website] and [link to FDA website].

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, But Under Specific Usage Guidelines. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Alison Brie And Dave Francos Together A 17 Million Copyright Battle Brews
May 20, 2025
Alison Brie And Dave Francos Together A 17 Million Copyright Battle Brews
May 20, 2025 -
 Final Liga Mx 2025 Toluca Vs America Informacion Crucial Sobre Fecha Y Hora
May 20, 2025
Final Liga Mx 2025 Toluca Vs America Informacion Crucial Sobre Fecha Y Hora
May 20, 2025 -
 Facial Differences And Discrimination A Cafe Customers Story
May 20, 2025
Facial Differences And Discrimination A Cafe Customers Story
May 20, 2025 -
 Pectra Upgrade Fuels Ethereum Investment 200 Million In New Funds
May 20, 2025
Pectra Upgrade Fuels Ethereum Investment 200 Million In New Funds
May 20, 2025 -
 America Vs Mohamed Recordando La Ultima Derrota En Una Final
May 20, 2025
America Vs Mohamed Recordando La Ultima Derrota En Una Final
May 20, 2025
Latest Posts
-
 A Candid Conversation Jamie Lee Curtis Reflects On Her Relationship With Lindsay Lohan
May 20, 2025
A Candid Conversation Jamie Lee Curtis Reflects On Her Relationship With Lindsay Lohan
May 20, 2025 -
 May 15th Brings New Warbond Drops To Helldivers 2s Masters Of Ceremony
May 20, 2025
May 15th Brings New Warbond Drops To Helldivers 2s Masters Of Ceremony
May 20, 2025 -
 Back To Back Murders Colombian Model And Mexican Influencers Deaths Fuel Femicide Outrage
May 20, 2025
Back To Back Murders Colombian Model And Mexican Influencers Deaths Fuel Femicide Outrage
May 20, 2025 -
 Putins Calculated Snub Demonstrating Independence From Trump
May 20, 2025
Putins Calculated Snub Demonstrating Independence From Trump
May 20, 2025 -
 Helldivers 2 Warbond Drop Masters Of Ceremony Update On May 15th
May 20, 2025
Helldivers 2 Warbond Drop Masters Of Ceremony Update On May 15th
May 20, 2025
