Novavax COVID-19 Vaccine Gets FDA Nod, But With Significant Caveats
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
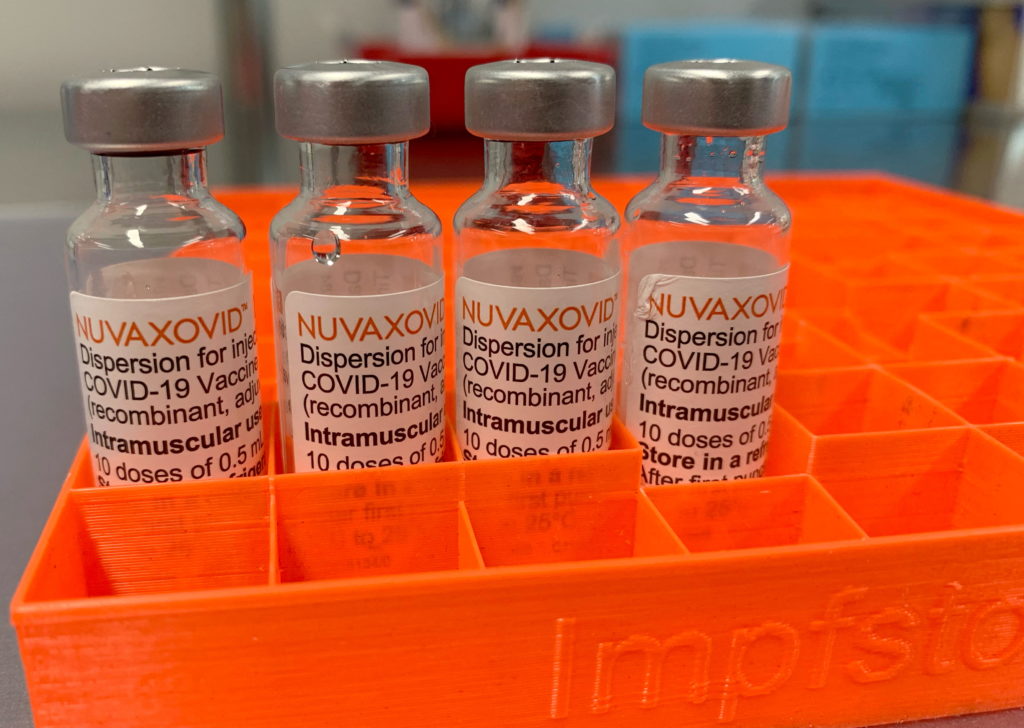
Novavax COVID-19 Vaccine Gets FDA Nod, but with Significant Caveats
The FDA has finally granted Emergency Use Authorization (EUA) to the Novavax COVID-19 vaccine, Nuvaxovid. This marks a significant development in the fight against the pandemic, offering an alternative vaccine option for those hesitant to receive mRNA-based vaccines. However, the authorization comes with notable caveats that temper initial celebratory reactions. The decision, announced [Insert Date of Announcement], has sparked both excitement and cautious optimism within the medical community and the general public.
A New Option, but Not a Game Changer:
The approval of the Novavax vaccine is undoubtedly a win for vaccine diversity. Unlike the Pfizer-BioNTech and Moderna vaccines which utilize mRNA technology, Nuvaxovid employs a more traditional protein subunit technology. This technology has been used in vaccines for decades, potentially appealing to individuals concerned about the newer mRNA approach. The protein subunit vaccines work by introducing a harmless piece of the virus's protein to the body, triggering an immune response.
However, the FDA's authorization is not without its limitations. The vaccine's efficacy, while deemed acceptable, lags behind that of the mRNA vaccines. Clinical trials showed a lower efficacy rate against the original COVID-19 strain, raising questions about its effectiveness against newer variants like Omicron and its subvariants. Furthermore, the approval is limited to individuals 18 years of age and older. [Insert Citation to FDA approval document or press release].
Caveats and Concerns:
Several significant caveats accompanied the FDA's EUA:
- Lower Efficacy: Studies have shown Nuvaxovid to have lower efficacy compared to mRNA vaccines, particularly against newer variants. This raises concerns about its overall effectiveness in curbing the spread of COVID-19 in the current epidemiological landscape.
- Limited Availability: The rollout of the Novavax vaccine is expected to be slower than previous vaccine rollouts due to production challenges and logistical hurdles. This could limit its immediate impact on vaccination rates.
- Potential Side Effects: While generally well-tolerated, the vaccine has been associated with some common side effects, including pain at the injection site, fatigue, headache, and muscle aches. These are generally mild and short-lived, but it's crucial for individuals to be aware of potential reactions. [Insert Link to CDC information on vaccine side effects].
- Variant Effectiveness: The efficacy of Nuvaxovid against currently circulating variants remains a crucial point of ongoing research and monitoring. This necessitates continued vigilance in evaluating its long-term effectiveness and the need for potential booster shots.
What This Means for the Future of COVID-19 Vaccination:
The approval of the Novavax vaccine presents a nuanced situation. While it offers an alternative technology for those hesitant about mRNA vaccines, its lower efficacy and logistical challenges might limit its overall impact. The vaccine’s role will likely be more prominent in specific populations or regions where access to mRNA vaccines is limited or where vaccine hesitancy based on technology is a major barrier.
The long-term effectiveness of Nuvaxovid against emerging variants will be closely monitored. Further research and clinical trials are vital to understand its complete efficacy profile and long-term benefits. The FDA's authorization signifies progress in diversifying vaccine options but underscores the ongoing need for comprehensive vaccination strategies and continued vigilance against the evolving COVID-19 virus.
Call to Action: Consult your healthcare provider to discuss which COVID-19 vaccine is best suited for your individual needs and health circumstances. Stay informed about the latest updates on COVID-19 vaccines and public health recommendations through reputable sources such as the CDC and WHO.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, But With Significant Caveats. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Jenn Sterger Details The Emotional Toll Of The Brett Favre Sexting Scandal
May 21, 2025
Jenn Sterger Details The Emotional Toll Of The Brett Favre Sexting Scandal
May 21, 2025 -
 Strip The Duck Analyzing Jon Jones Latest Comments On Tom Aspinall
May 21, 2025
Strip The Duck Analyzing Jon Jones Latest Comments On Tom Aspinall
May 21, 2025 -
 Confirmed A New Peaky Blinders Series Is Coming Expect Big Changes
May 21, 2025
Confirmed A New Peaky Blinders Series Is Coming Expect Big Changes
May 21, 2025 -
 Cnn Reports Israeli Strikes Hit Only Remaining Hospital In Northern Gaza
May 21, 2025
Cnn Reports Israeli Strikes Hit Only Remaining Hospital In Northern Gaza
May 21, 2025 -
 Jamie Lee Curtis Shares Update On Her Bond With Lindsay Lohan Following Freaky Friday
May 21, 2025
Jamie Lee Curtis Shares Update On Her Bond With Lindsay Lohan Following Freaky Friday
May 21, 2025
Latest Posts
-
 Linekers Controversial Tweets The Fallout And Potential Match Of The Day Departure
May 21, 2025
Linekers Controversial Tweets The Fallout And Potential Match Of The Day Departure
May 21, 2025 -
 Gary Linekers Bbc Departure Whats Next For Match Of The Day Host
May 21, 2025
Gary Linekers Bbc Departure Whats Next For Match Of The Day Host
May 21, 2025 -
 Watch Now A Powerful Wwi Drama Featuring Daniel Craig Cillian Murphy And Tom Hardy
May 21, 2025
Watch Now A Powerful Wwi Drama Featuring Daniel Craig Cillian Murphy And Tom Hardy
May 21, 2025 -
 Jenn Sterger Speaks Out The Fallout From The Brett Favre Sexting Scandal
May 21, 2025
Jenn Sterger Speaks Out The Fallout From The Brett Favre Sexting Scandal
May 21, 2025 -
 Jon Jones Cryptic I M Done Message Ufc Career Hanging In The Balance After Aspinall Stalemate
May 21, 2025
Jon Jones Cryptic I M Done Message Ufc Career Hanging In The Balance After Aspinall Stalemate
May 21, 2025
