Novavax COVID-19 Vaccine Gets FDA Nod, But With Significant Usage Constraints
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Gets FDA Nod, but with Significant Usage Constraints
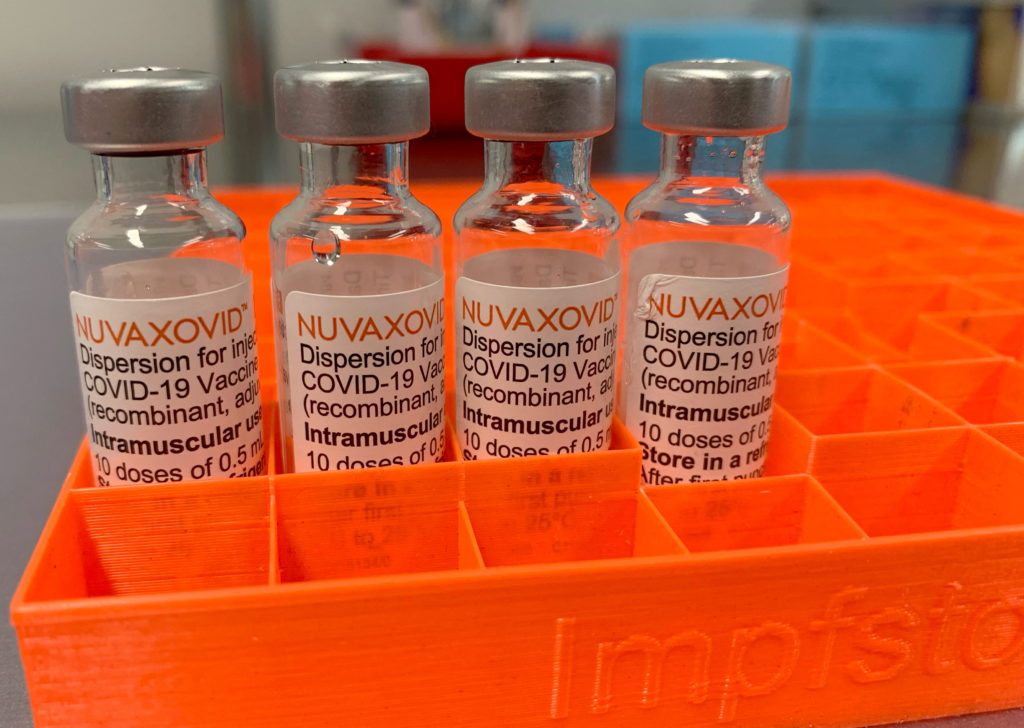
The FDA has finally approved the Novavax COVID-19 vaccine, Nuvaxovid, but its rollout faces significant hurdles due to limited demand and supply chain issues. This approval, coming over two years after the initial emergency authorization of other vaccines, marks a significant milestone, yet its impact might be less dramatic than initially anticipated.
The authorization covers individuals 18 years and older. While the news is positive for those seeking a protein-subunit vaccine alternative, the reality is that the vaccine's arrival might be too late to significantly alter the current vaccination landscape in the United States. Let's examine the reasons why.
Limited Demand in a Post-Pandemic World
The initial excitement surrounding the Novavax vaccine has largely waned. The widespread availability of mRNA vaccines like Pfizer-BioNTech and Moderna, coupled with the decreased severity of COVID-19 infections, has significantly reduced public demand for a new vaccine. Many individuals have already received their primary series and booster shots, leaving a smaller pool of potential recipients for Nuvaxovid.
This diminished demand poses a significant challenge to Novavax, potentially impacting its ability to recoup its substantial investment in research and development. The company will need to implement a robust marketing strategy to effectively reach target audiences and convince them of the vaccine's benefits.
Supply Chain Bottlenecks and Production Challenges
Beyond the issue of demand, Novavax has also grappled with considerable production challenges. Initial production targets were not met, further delaying the vaccine's widespread availability. These supply chain issues, coupled with the decreased demand, create a complex scenario that may hinder the vaccine's potential impact.
Experts are questioning whether the current manufacturing capacity is sufficient to justify the resources invested in obtaining FDA approval. This raises concerns about the vaccine's long-term viability and its contribution to overall vaccination efforts.
Nuvaxovid: A Different Approach
It's crucial to remember that Nuvaxovid employs a different technology compared to the mRNA vaccines. It utilizes a protein-subunit approach, making it an attractive option for individuals hesitant about mRNA technology. This technology has been used for decades in other vaccines, potentially reassuring some vaccine-hesitant populations.
- How Nuvaxovid Works: The vaccine uses a harmless piece of the COVID-19 virus to stimulate an immune response. This differs from the mRNA vaccines, which deliver instructions to cells to create a viral protein. This difference may be a key factor for some individuals making their vaccine choice.
The Road Ahead for Novavax
The FDA approval is undoubtedly a victory for Novavax, but the road ahead remains challenging. The company needs to address the supply chain issues, implement effective marketing strategies to target the remaining unvaccinated population, and potentially focus on international markets where demand may be higher.
While Nuvaxovid offers an alternative technology, its late entry into the market and the complexities surrounding its production and distribution limit its immediate impact on the COVID-19 pandemic. The long-term success of this vaccine will depend on Novavax's ability to overcome these significant challenges.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein-subunit vaccine, vaccine hesitancy, supply chain, vaccine rollout, mRNA vaccine, pandemic, public health.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, But With Significant Usage Constraints. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Snls 50th Season Ends On A High Note Record Viewership
May 20, 2025
Snls 50th Season Ends On A High Note Record Viewership
May 20, 2025 -
 You Wont Believe This The Funniest Sex Scene Of The Year Is In Overcompensating
May 20, 2025
You Wont Believe This The Funniest Sex Scene Of The Year Is In Overcompensating
May 20, 2025 -
 Russia Ukraine Conflict Trump Intervenes Peace Talks To Begin
May 20, 2025
Russia Ukraine Conflict Trump Intervenes Peace Talks To Begin
May 20, 2025 -
 Urgent Security Breach Private And Criminal Records Stolen From Legal Aid
May 20, 2025
Urgent Security Breach Private And Criminal Records Stolen From Legal Aid
May 20, 2025 -
 High Stakes Testimony Will Cassie Venturas Account Convict Sean Diddy Combs
May 20, 2025
High Stakes Testimony Will Cassie Venturas Account Convict Sean Diddy Combs
May 20, 2025
Latest Posts
-
 Over 5 Billion Inflows Bitcoin Etf Investment Surges
May 21, 2025
Over 5 Billion Inflows Bitcoin Etf Investment Surges
May 21, 2025 -
 New Guidelines Aim To Improve Tourist Conduct In Bali
May 21, 2025
New Guidelines Aim To Improve Tourist Conduct In Bali
May 21, 2025 -
 Joe Bidens Prostate Cancer What We Know And What It Means
May 21, 2025
Joe Bidens Prostate Cancer What We Know And What It Means
May 21, 2025 -
 New Clues Emerge From A Canadian Pachyrhinosaurus Fossil Bed
May 21, 2025
New Clues Emerge From A Canadian Pachyrhinosaurus Fossil Bed
May 21, 2025 -
 Record Ratings Snl Concludes 50th Season On A High Note
May 21, 2025
Record Ratings Snl Concludes 50th Season On A High Note
May 21, 2025
