Novavax COVID-19 Vaccine Gets FDA Nod, But With Unusual Usage Constraints
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Gets FDA Nod, but with Unusual Usage Constraints
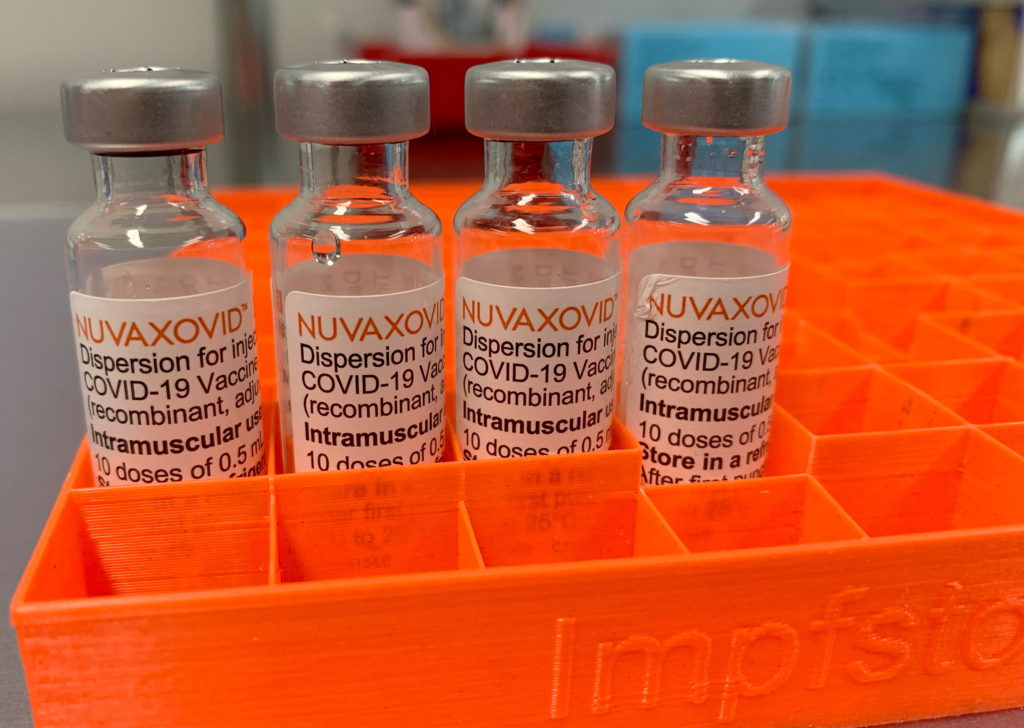
The FDA has finally approved the Novavax COVID-19 vaccine, Nuvaxovid, but its authorization comes with significant caveats that limit its potential impact. This long-awaited approval, announced [Insert Date of Announcement], marks a milestone for the vaccine landscape, but the unusual restrictions raise questions about its practical use in the ongoing pandemic.
The approval of Nuvaxovid, a protein-subunit vaccine different from the mRNA vaccines already widely available, was initially met with cautious optimism. Many hoped it would provide an alternative for individuals hesitant to receive mRNA vaccines due to concerns about their novel technology. However, the FDA's decision to authorize it only for specific populations under specific conditions dampens this enthusiasm.
Limited Authorization: A Closer Look at the Restrictions
The FDA's authorization is not a blanket approval. The agency has placed significant limitations on its use, primarily focusing on:
- Specific Age Groups: The vaccine is currently authorized only for adults 18 years and older. This excludes a significant portion of the population who may benefit from a different vaccine option. Further studies are needed to determine its safety and efficacy in younger age groups.
- Primary Series Only: Currently, the FDA has only approved Nuvaxovid for the initial two-dose primary vaccination series. There is no authorization for booster doses at this time. This significantly limits its utility in a landscape where booster shots are widely recommended.
- Conditional Approval: The authorization is considered conditional, meaning ongoing safety monitoring and further data collection are required. This suggests a degree of uncertainty surrounding its long-term effectiveness and potential side effects.
These constraints raise concerns about the vaccine's overall impact. With mRNA vaccines readily available and already authorized for wider use, including booster doses, the limited scope of Nuvaxovid's approval raises questions about its practical application.
Why the Restricted Approval?
While the FDA hasn't explicitly stated the reasons for these limitations, several factors likely contributed:
- Lower Efficacy Data: While Nuvaxovid demonstrated efficacy in clinical trials, its effectiveness might be comparatively lower than the established mRNA vaccines against currently circulating variants.
- Production Challenges: The manufacturing process for Nuvaxovid might present challenges in scaling up production to meet potential demand.
- Safety Data Limitations: While deemed safe, the available safety data may not be as extensive as that available for mRNA vaccines, leading to a more cautious approach.
The Future of Novavax's COVID-19 Vaccine
The future of Nuvaxovid remains uncertain. The FDA's conditional approval suggests a path for expansion, provided further data supports broadening its use. Further clinical trials focusing on younger age groups and booster doses are crucial. Novavax will need to address the limitations highlighted by the FDA to increase its vaccine's impact. The company's ability to overcome these hurdles and demonstrate greater efficacy against emerging variants will determine the vaccine's ultimate role in the global COVID-19 vaccination strategy.
This development highlights the dynamic nature of vaccine development and approval. The focus now shifts to further research and data collection to determine the true potential of Nuvaxovid in the fight against COVID-19. The FDA's approach underscores the importance of rigorous scientific evaluation and a cautious approach to vaccine deployment. We will continue to monitor developments and provide updates as more information becomes available.
Keywords: Novavax, COVID-19 vaccine, Nuvaxovid, FDA approval, vaccine authorization, protein-subunit vaccine, mRNA vaccine, vaccine efficacy, vaccine safety, conditional approval, booster dose, pandemic, public health.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, But With Unusual Usage Constraints. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 New Rules For Tourists In Bali Curbing Misbehavior And Protecting The Island
May 21, 2025
New Rules For Tourists In Bali Curbing Misbehavior And Protecting The Island
May 21, 2025 -
 Ellen De Generes Emotional Return To Social Media A Fans Perspective
May 21, 2025
Ellen De Generes Emotional Return To Social Media A Fans Perspective
May 21, 2025 -
 Alito And Roberts Supreme Court Service A Look Back At Two Decades
May 21, 2025
Alito And Roberts Supreme Court Service A Look Back At Two Decades
May 21, 2025 -
 Gaza Healthcare Crisis Deepens Israeli Strikes Hit Only Remaining Northern Hospital
May 21, 2025
Gaza Healthcare Crisis Deepens Israeli Strikes Hit Only Remaining Northern Hospital
May 21, 2025 -
 Isolated Strong Storms Possible Late Tuesday Night
May 21, 2025
Isolated Strong Storms Possible Late Tuesday Night
May 21, 2025
Latest Posts
-
 Police Investigate Church Break In Report Of Defecation
May 21, 2025
Police Investigate Church Break In Report Of Defecation
May 21, 2025 -
 Trump Doj And James A Complex Web Of Legal Battles
May 21, 2025
Trump Doj And James A Complex Web Of Legal Battles
May 21, 2025 -
 Diplomatic Tensions Rise As Allies Demand End To Israels Gaza Operation
May 21, 2025
Diplomatic Tensions Rise As Allies Demand End To Israels Gaza Operation
May 21, 2025 -
 New Orleans Jailbreak Fourth Inmate Captured As District Attorneys Staff Seek Safety
May 21, 2025
New Orleans Jailbreak Fourth Inmate Captured As District Attorneys Staff Seek Safety
May 21, 2025 -
 Police Teenagers Arrested For Urinating And Defecating In Santa Rosa Church
May 21, 2025
Police Teenagers Arrested For Urinating And Defecating In Santa Rosa Church
May 21, 2025
