Novavax COVID-19 Vaccine Gets FDA Nod, Strict Usage Guidelines Announced
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
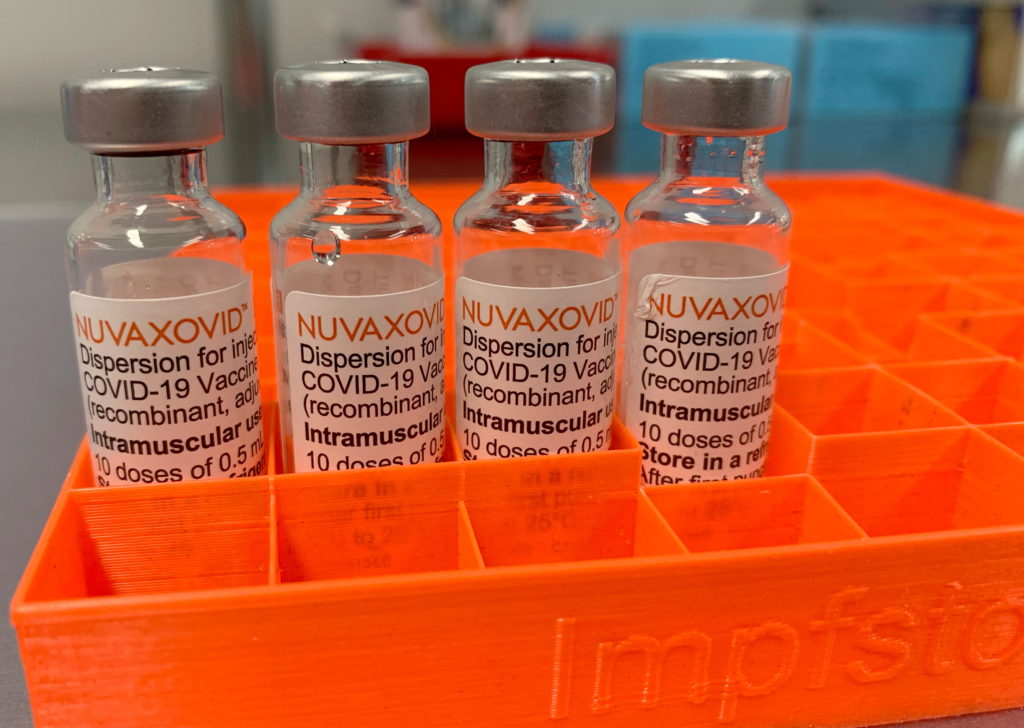
Novavax COVID-19 Vaccine Gets FDA Nod, But with Strict Usage Guidelines
The FDA has finally granted Emergency Use Authorization (EUA) to Novavax's COVID-19 vaccine, Nuvaxovid, offering a new option for individuals hesitant about mRNA vaccines. However, the authorization comes with strict usage guidelines, limiting its immediate impact. This development marks a significant milestone in the fight against the pandemic, but its overall effect remains to be seen.
What is Nuvaxovid and Why is it Different?
Unlike the widely used Pfizer-BioNTech and Moderna vaccines, which employ mRNA technology, Nuvaxovid is a protein subunit vaccine. This means it uses a piece of the virus's protein to trigger an immune response. This technology has been used for decades in vaccines against diseases like hepatitis B and influenza, potentially making it more appealing to those wary of newer mRNA technology. Many individuals have expressed concerns regarding the speed of mRNA vaccine development; Nuvaxovid's traditional approach may address these concerns for some.
FDA Approval: A Cautious Welcome
While the FDA's approval is a victory for Novavax, the agency's accompanying guidelines highlight the complexities of the situation. The EUA is not a blanket approval for widespread use. The FDA emphasizes that Nuvaxovid should primarily be used in specific circumstances and for targeted populations. This targeted approach is a key factor in understanding the vaccine's immediate impact.
Strict Usage Guidelines: Key Considerations
The FDA's stringent guidelines include:
- Limited Availability: Initially, the vaccine will have limited availability, potentially impacting its immediate rollout and widespread adoption.
- Specific Populations: The FDA may initially recommend Nuvaxovid for specific demographics or individuals who haven't received other vaccines due to concerns or previous adverse reactions. This focused approach ensures careful monitoring and risk assessment.
- Ongoing Monitoring: The FDA will continue to closely monitor the vaccine's safety and efficacy post-authorization. This ongoing surveillance is critical for assessing long-term effects and making any necessary adjustments to its usage.
Implications for the Future of COVID-19 Vaccination
The arrival of Nuvaxovid offers a potential alternative for individuals hesitant about mRNA vaccines, potentially increasing vaccination rates. However, the limited initial availability and targeted usage guidelines mean that its immediate impact on overall COVID-19 vaccination levels may be less dramatic than initially hoped. The long-term success of Nuvaxovid will depend on factors like production capacity, distribution efficiency, and ongoing public health messaging.
Addressing Public Concerns and Misinformation
Accurate and transparent communication regarding the vaccine's efficacy, safety profile, and availability is crucial to building public trust and ensuring its successful implementation. Combating misinformation about the vaccine through reliable sources and public health initiatives will be essential. The CDC website and other reputable sources should be consulted for the latest information.
Conclusion: A Step Forward, But More to Come
The FDA's approval of the Novavax COVID-19 vaccine is a significant step forward in the fight against the pandemic. However, the accompanying strict usage guidelines highlight the need for a cautious and targeted approach. The vaccine's long-term impact will depend on various factors, including production scale, distribution strategies, and public perception. Continued monitoring and transparent communication will be key to maximizing its potential benefits. We encourage you to consult your healthcare provider to determine the best vaccination strategy for you.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, Strict Usage Guidelines Announced. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Potholes And Overflowing Bins Government Considers Using Criminal Labor
May 20, 2025
Potholes And Overflowing Bins Government Considers Using Criminal Labor
May 20, 2025 -
 Record Ratings Snl Concludes Historic 50th Season
May 20, 2025
Record Ratings Snl Concludes Historic 50th Season
May 20, 2025 -
 Potholes And Overflowing Bins Government Considers Criminal Workforce Solution
May 20, 2025
Potholes And Overflowing Bins Government Considers Criminal Workforce Solution
May 20, 2025 -
 Pacifier Weaning When To Stop And How To Help Your Child
May 20, 2025
Pacifier Weaning When To Stop And How To Help Your Child
May 20, 2025 -
 Nyc Incident Mexican Navy Ship Impacts Brooklyn Bridge
May 20, 2025
Nyc Incident Mexican Navy Ship Impacts Brooklyn Bridge
May 20, 2025
Latest Posts
-
 Cnn Reports Israeli Strikes Hit Only Remaining Hospital In North Gaza
May 20, 2025
Cnn Reports Israeli Strikes Hit Only Remaining Hospital In North Gaza
May 20, 2025 -
 Eu Uk Brexit Talks A Delicate Balance On The Brink Of Collapse
May 20, 2025
Eu Uk Brexit Talks A Delicate Balance On The Brink Of Collapse
May 20, 2025 -
 The Real Story Behind Jamie Lee Curtis And Lindsay Lohans Bond
May 20, 2025
The Real Story Behind Jamie Lee Curtis And Lindsay Lohans Bond
May 20, 2025 -
 Peaky Blinders Creator Reveals Plans For A New Series And A Significant Shift
May 20, 2025
Peaky Blinders Creator Reveals Plans For A New Series And A Significant Shift
May 20, 2025 -
 Bold Bets Drive 5 B Influx Into Bitcoin Etfs Market Analysis And Predictions
May 20, 2025
Bold Bets Drive 5 B Influx Into Bitcoin Etfs Market Analysis And Predictions
May 20, 2025
