Novavax COVID-19 Vaccine Gets FDA Nod, Under Stricter Guidelines
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
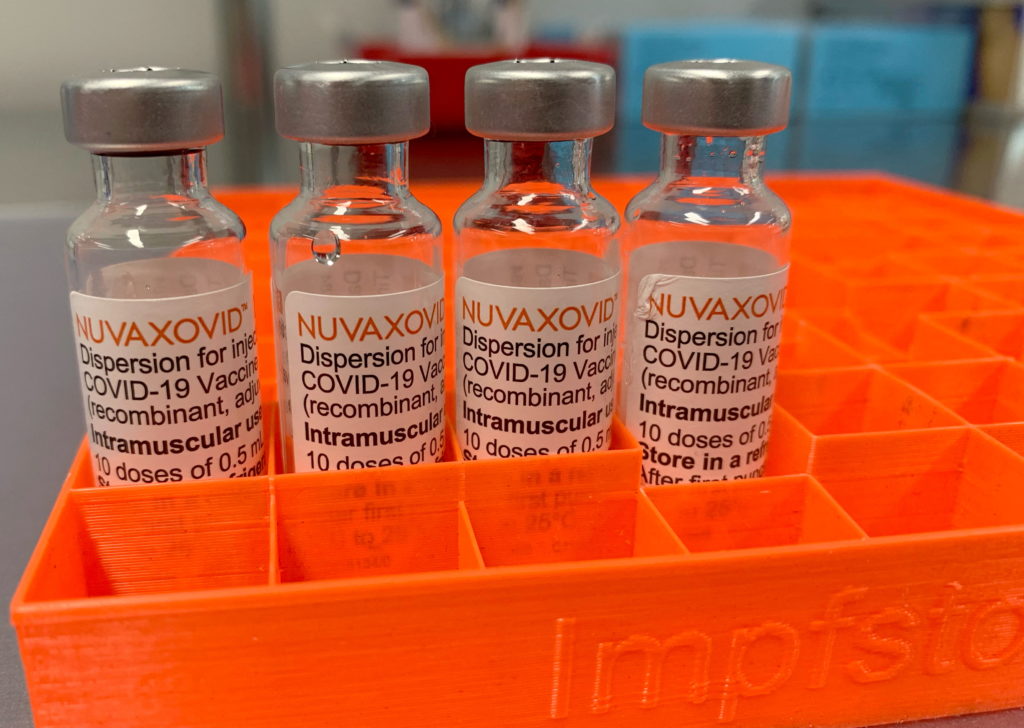
Novavax COVID-19 Vaccine Gets FDA Nod, But Under Stricter Guidelines
The FDA has finally granted Emergency Use Authorization (EUA) to the Novavax COVID-19 vaccine, Nuvaxovid, marking a significant development in the fight against the pandemic. However, the approval comes with stricter guidelines than initially anticipated, raising questions about its widespread adoption and impact on vaccination efforts.
This long-awaited authorization provides another option for individuals seeking COVID-19 protection, particularly those hesitant about mRNA vaccines like Pfizer-BioNTech and Moderna. Novavax's protein-subunit technology, a more traditional approach, has been touted as a potentially appealing alternative for this population. But the FDA's decision to impose stricter guidelines underscores the need for ongoing vigilance and rigorous safety monitoring.
What Makes the Novavax Approval Different?
Unlike the relatively straightforward EUA process for mRNA vaccines, the Novavax approval comes with several key caveats:
- Limited Age Range: Initially, the EUA is only for individuals 18 years of age and older. Further studies are needed to determine its safety and efficacy in younger populations. This contrasts with the broader age ranges covered by the Pfizer and Moderna vaccines.
- Stringent Monitoring Requirements: The FDA will be closely monitoring the vaccine's safety profile post-authorization. This heightened surveillance reflects concerns raised during clinical trials and emphasizes the need for continuous data collection and analysis.
- Specific Dosage and Administration: The FDA has outlined precise dosage requirements and administration protocols, leaving less room for flexibility compared to other authorized vaccines. This strict adherence to guidelines aims to minimize potential risks.
- Production and Supply Chain: The initial rollout of the Novavax vaccine is expected to be more limited than the existing mRNA vaccines, potentially affecting its immediate availability. Concerns around production capacity and distribution logistics may impact the vaccine's reach.
Why the Stricter Guidelines?
The stricter guidelines likely stem from several factors:
- Clinical Trial Data: While the clinical trials showed the vaccine's effectiveness in preventing COVID-19, some safety concerns were raised, necessitating a more cautious approach by the FDA. This is a standard procedure for new vaccines, and doesn't necessarily imply higher risks.
- Emerging Variants: The ever-evolving nature of the SARS-CoV-2 virus, and the emergence of new variants, adds another layer of complexity. The FDA's stringent requirements may reflect a need for better understanding of the vaccine's effectiveness against these variants.
- Public Confidence: Maintaining public trust in vaccines is crucial. The FDA's cautious approach may be a deliberate strategy to build confidence in the Novavax vaccine, particularly given the existing vaccine hesitancy among some segments of the population.
What Does This Mean for the Future of COVID-19 Vaccination?
The Novavax vaccine's approval, even with stricter guidelines, presents a valuable addition to the existing arsenal of COVID-19 vaccines. Its different technology may help increase vaccine uptake among hesitant individuals. However, the limited initial availability and stringent monitoring requirements will likely impact its immediate influence on overall vaccination rates. The FDA’s decision highlights the importance of continued research, rigorous safety monitoring, and transparent communication to ensure public confidence in all COVID-19 vaccines. Further updates and information will be vital as the rollout progresses. Stay informed by following reputable sources like the and the .
Call to Action: Consult your healthcare provider to discuss which COVID-19 vaccine is right for you. Staying informed about COVID-19 vaccination is crucial for protecting yourself and your community.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, Under Stricter Guidelines. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Uk Government Considers Employing Offenders For Pothole Filling And Waste Collection
May 20, 2025
Uk Government Considers Employing Offenders For Pothole Filling And Waste Collection
May 20, 2025 -
 New Rules Target Buy Now Pay Later Risks Enhanced Consumer Safeguards
May 20, 2025
New Rules Target Buy Now Pay Later Risks Enhanced Consumer Safeguards
May 20, 2025 -
 St Louis Rebuilds Days After Devastating Tornado
May 20, 2025
St Louis Rebuilds Days After Devastating Tornado
May 20, 2025 -
 Jon Jones Ufc Future Uncertain Aspinall Talks Stall Fans React To Cryptic Hint
May 20, 2025
Jon Jones Ufc Future Uncertain Aspinall Talks Stall Fans React To Cryptic Hint
May 20, 2025 -
 Revive El Momento Yahir Y Victor Garcia Cantan Otra Vez En Juego De Voces
May 20, 2025
Revive El Momento Yahir Y Victor Garcia Cantan Otra Vez En Juego De Voces
May 20, 2025
Latest Posts
-
 Brett Favre Sexting Scandal Jenn Sterger Recounts Her Experience And Its Aftermath
May 20, 2025
Brett Favre Sexting Scandal Jenn Sterger Recounts Her Experience And Its Aftermath
May 20, 2025 -
 New Wwi Movie Featuring Daniel Craig Cillian Murphy And Tom Hardy Streaming Now
May 20, 2025
New Wwi Movie Featuring Daniel Craig Cillian Murphy And Tom Hardy Streaming Now
May 20, 2025 -
 Peaky Blinders Creator Announces New Series Detailing Key Departure
May 20, 2025
Peaky Blinders Creator Announces New Series Detailing Key Departure
May 20, 2025 -
 Controversy Erupts Jon Jones And The Ufcs Handling Of Aspinall Injury
May 20, 2025
Controversy Erupts Jon Jones And The Ufcs Handling Of Aspinall Injury
May 20, 2025 -
 Post Pectra Upgrade Investors Inject 200 Million Into Ethereum Funds
May 20, 2025
Post Pectra Upgrade Investors Inject 200 Million Into Ethereum Funds
May 20, 2025
