Novavax COVID-19 Vaccine Gets FDA Nod, Use Restricted: What You Need To Know
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
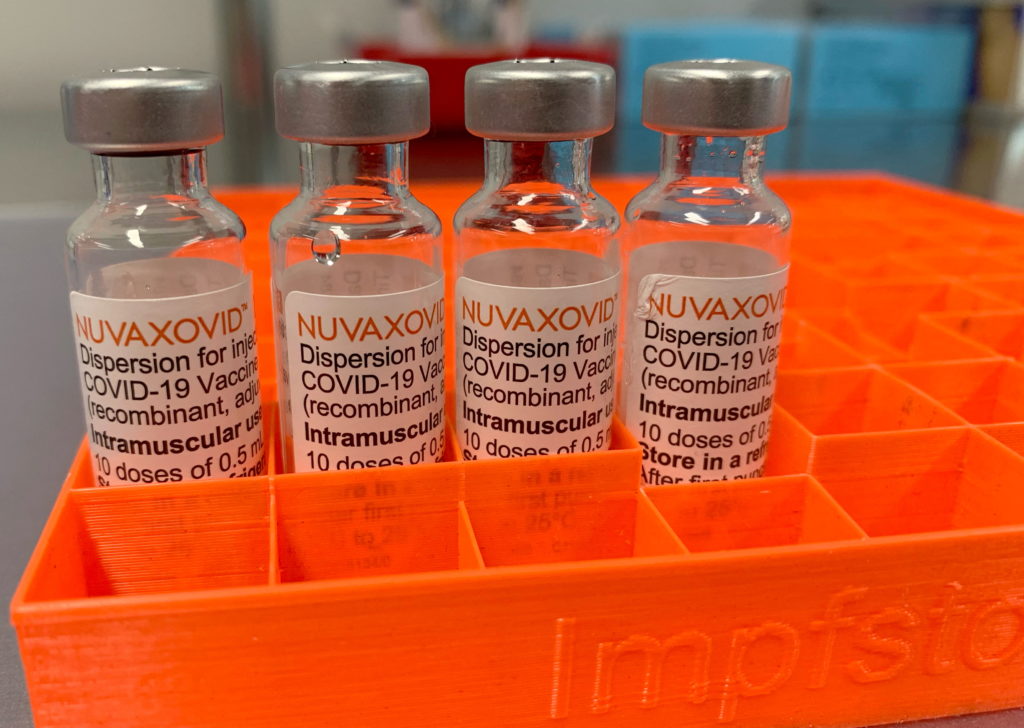
Novavax COVID-19 Vaccine Gets FDA Nod, But Use Remains Restricted: What You Need to Know
The Novavax COVID-19 vaccine, Nuvaxovid, has finally received full approval from the U.S. Food and Drug Administration (FDA), marking a significant milestone in the fight against the pandemic. However, this approval comes with caveats. While a victory for Novavax and a potential boost to vaccination rates, the FDA's authorization isn't a complete green light, and its use remains restricted. Let's delve into the details.
What does full FDA approval mean?
Full FDA approval signifies that the agency has completed a rigorous review of the vaccine's safety and efficacy data, finding it to meet their high standards. This is a step up from the previous Emergency Use Authorization (EUA), providing a higher level of assurance for both healthcare providers and the public. The approval solidifies the vaccine's place in the arsenal of COVID-19 prevention tools.
So, why the restrictions?
Despite full approval, the FDA has placed limitations on the distribution and use of the Novavax vaccine. This is primarily due to the current landscape of COVID-19. With other vaccines readily available and widely used, the demand for Novavax might be lower. The FDA's decision likely considers factors such as:
- Current vaccination rates: A significant portion of the eligible population has already received other COVID-19 vaccines.
- Vaccine availability: Existing vaccines are readily available and accessible, making the immediate need for a new vaccine less pressing.
- Variant effectiveness: While effective against earlier variants, the efficacy of the Novavax vaccine against newer variants might warrant further study and monitoring. This aspect requires continuous evaluation in light of the virus's ongoing evolution. ()
Who is the Novavax vaccine for?
The Novavax vaccine is authorized for individuals 18 years of age and older. While full approval is a positive development, it's crucial to consult with your healthcare provider to determine if the Novavax vaccine is the right choice for you, considering your individual health circumstances and pre-existing conditions. They can guide you based on your specific needs and the availability of other vaccines in your area.
What are the next steps?
Novavax and the FDA will continue to monitor the vaccine's safety and effectiveness following full approval. This ongoing surveillance is essential to ensure the vaccine's long-term efficacy and safety profile remains consistent. Further research into its performance against newer variants is also anticipated.
The Bottom Line:
The FDA's full approval of the Novavax COVID-19 vaccine is a significant step forward. However, its restricted use reflects the current epidemiological context and the availability of other effective vaccines. While this approval adds another tool to the fight against COVID-19, it's crucial to understand the nuances of its limited distribution and consult with healthcare professionals for personalized guidance on vaccination strategies. This ensures that individuals make informed decisions based on their specific health profiles and access to available vaccines.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, vaccine safety, vaccine efficacy, COVID-19 variants, vaccination rates, vaccine authorization, emergency use authorization, EUA, healthcare provider, vaccine recommendations.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, Use Restricted: What You Need To Know. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Mlb Betting Predictions Greg Petersons May 18th Selections
May 20, 2025
Mlb Betting Predictions Greg Petersons May 18th Selections
May 20, 2025 -
 Is Bare Beating On Public Transport Getting Out Of Hand
May 20, 2025
Is Bare Beating On Public Transport Getting Out Of Hand
May 20, 2025 -
 Weaning Your Child Off Pacifiers And Thumbs A Practical Approach
May 20, 2025
Weaning Your Child Off Pacifiers And Thumbs A Practical Approach
May 20, 2025 -
 Mlb Best Bets May 18 Greg Petersons Baseball Predictions
May 20, 2025
Mlb Best Bets May 18 Greg Petersons Baseball Predictions
May 20, 2025 -
 Walking With Dinosaurs Investigating A Canadian Pachyrhinosaurus Fossil Site
May 20, 2025
Walking With Dinosaurs Investigating A Canadian Pachyrhinosaurus Fossil Site
May 20, 2025
Latest Posts
-
 Israeli Airstrikes Hit Only Remaining Hospital In Northern Gaza
May 20, 2025
Israeli Airstrikes Hit Only Remaining Hospital In Northern Gaza
May 20, 2025 -
 Family Struck By Train On Bridge Two Fatalities One Child Missing Another Hurt
May 20, 2025
Family Struck By Train On Bridge Two Fatalities One Child Missing Another Hurt
May 20, 2025 -
 Thumb Sucking And Pacifiers A Guide For Parents On When To Wean
May 20, 2025
Thumb Sucking And Pacifiers A Guide For Parents On When To Wean
May 20, 2025 -
 Gaza Hospital Destroyed Israeli Strikes Target Last Northern Facility
May 20, 2025
Gaza Hospital Destroyed Israeli Strikes Target Last Northern Facility
May 20, 2025 -
 St Louis Tornado Aftermath Stories Of Resilience And Community Support
May 20, 2025
St Louis Tornado Aftermath Stories Of Resilience And Community Support
May 20, 2025
