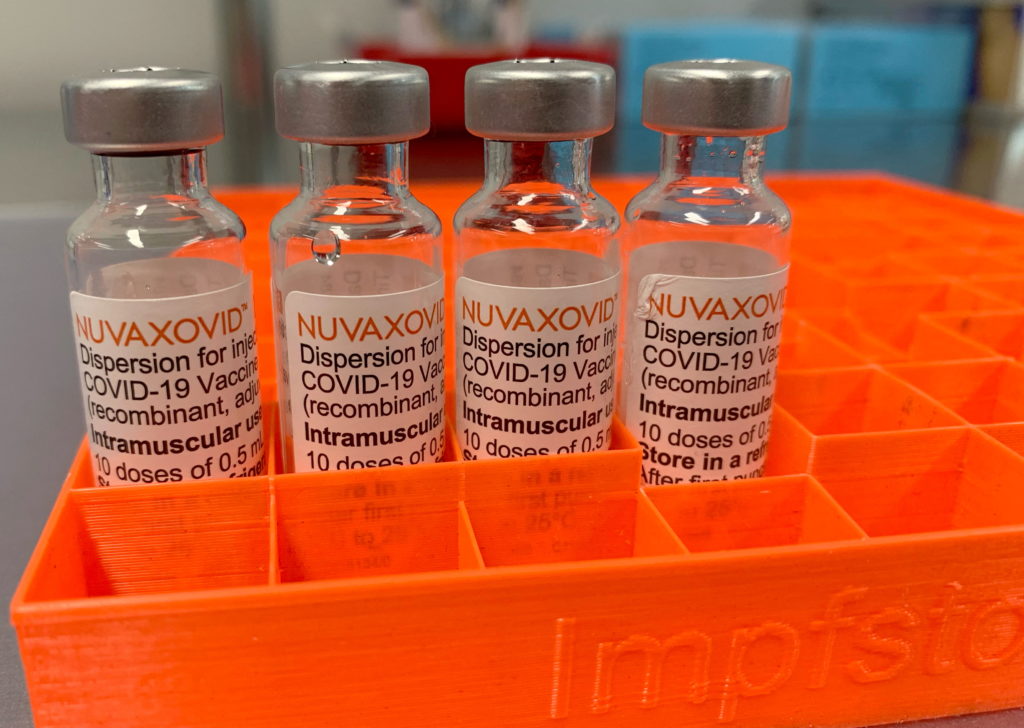
Novavax COVID-19 Vaccine Gets FDA Nod, With Unconventional Usage Constraints
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Gets FDA Nod, but with Unconventional Usage Constraints
The FDA has finally granted approval to the Novavax COVID-19 vaccine, Nuvaxovid, but its authorization comes with significant limitations, raising questions about its practical impact. This long-awaited approval marks a significant milestone in the fight against the pandemic, but the unconventional usage constraints imposed by the FDA may restrict its widespread adoption.
The approval, announced [Insert Date], applies to individuals 18 years of age and older. However, unlike other widely used COVID-19 vaccines, Nuvaxovid's use is explicitly limited. The FDA's authorization emphasizes its use as an alternative option for those who cannot or will not receive mRNA vaccines (like Pfizer-BioNTech and Moderna), or viral vector vaccines (like Johnson & Johnson). This targeted approach differs drastically from the broader rollout seen with other vaccines.
Why the Restricted Authorization?
The FDA's decision to grant a more limited authorization is likely due to several factors. While Nuvaxovid has demonstrated efficacy and safety in clinical trials, its arrival relatively late in the pandemic, after the widespread deployment of other vaccines, significantly impacts its potential market share. Furthermore, the overall efficacy data might not be as compelling compared to the already established vaccines. The agency also needs to carefully weigh the benefits against potential risks, particularly given the evolving COVID-19 landscape and the availability of other vaccines.
Understanding Nuvaxovid's Mechanism
Unlike mRNA vaccines that use messenger RNA to instruct cells to produce a viral protein, or viral vector vaccines that use a modified virus to deliver genetic material, Nuvaxovid employs a more traditional approach. It's a protein subunit vaccine, meaning it uses purified fragments of the SARS-CoV-2 virus to trigger an immune response. This technology has been used for decades in other vaccines, potentially offering an advantage for those hesitant about newer technologies.
What are the potential implications of these limitations?
The restricted authorization presents several challenges:
- Limited Demand: With other readily available and widely accepted vaccines, the demand for Nuvaxovid might be significantly lower than initially projected.
- Logistical Hurdles: Targeted distribution to specific populations requires additional logistical planning and resource allocation.
- Public Perception: The limited authorization might fuel existing vaccine hesitancy and contribute to confusion among the public.
Looking Ahead: The Future of Nuvaxovid
Despite the constraints, the FDA approval of Nuvaxovid provides a valuable addition to the global vaccine arsenal. It offers an alternative for individuals with specific concerns about mRNA or viral vector vaccines. However, the success of Nuvaxovid hinges on effective communication, targeted distribution strategies, and a clear understanding of its role within the existing vaccination landscape. Further research and data analysis will be crucial in assessing its long-term impact on the pandemic.
Further Reading:
- [Link to FDA's official press release on Nuvaxovid approval]
- [Link to CDC's information on COVID-19 vaccines]
This article provides valuable information about the newly approved Novavax COVID-19 vaccine, including its mechanism, limitations, and potential impact. While its limited authorization presents challenges, it offers a crucial alternative for some individuals. The future of Nuvaxovid will depend heavily on effective communication, strategic deployment, and ongoing monitoring of its efficacy and safety profile.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, With Unconventional Usage Constraints. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Mud Hens Edge Rail Riders With Early And Late Innings Scoring
May 20, 2025
Mud Hens Edge Rail Riders With Early And Late Innings Scoring
May 20, 2025 -
 The Visionarys Method How One Self Made Billionaire Creates Innovative Concepts
May 20, 2025
The Visionarys Method How One Self Made Billionaire Creates Innovative Concepts
May 20, 2025 -
 Always Kept It Real Jamie Lee Curtis On Her Relationship With Lindsay Lohan
May 20, 2025
Always Kept It Real Jamie Lee Curtis On Her Relationship With Lindsay Lohan
May 20, 2025 -
 Untold Brett Favre A J Perez Reveals Behind The Scenes Threats
May 20, 2025
Untold Brett Favre A J Perez Reveals Behind The Scenes Threats
May 20, 2025 -
 From Zero To Billions How This Visionary Generates Innovative Ideas
May 20, 2025
From Zero To Billions How This Visionary Generates Innovative Ideas
May 20, 2025
Latest Posts
-
 Post Pectra Upgrade Investors Pour 200 Million Into Ethereum
May 20, 2025
Post Pectra Upgrade Investors Pour 200 Million Into Ethereum
May 20, 2025 -
 Urgent Legal Aid Data Breach Reveals Sensitive Client Information
May 20, 2025
Urgent Legal Aid Data Breach Reveals Sensitive Client Information
May 20, 2025 -
 Freaky Friday Reunion Jamie Lee Curtis On Maintaining Contact With Lindsay Lohan
May 20, 2025
Freaky Friday Reunion Jamie Lee Curtis On Maintaining Contact With Lindsay Lohan
May 20, 2025 -
 Match Of The Day Host Gary Linekers Potential Bbc Exit Latest Updates
May 20, 2025
Match Of The Day Host Gary Linekers Potential Bbc Exit Latest Updates
May 20, 2025 -
 Days After St Louis Tornado Residents Rebuild Community Rallies
May 20, 2025
Days After St Louis Tornado Residents Rebuild Community Rallies
May 20, 2025
