Novavax Vaccine Gets FDA Nod, But Faces Unusual Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax Vaccine Gets FDA Nod, But Faces Unusual Usage Restrictions
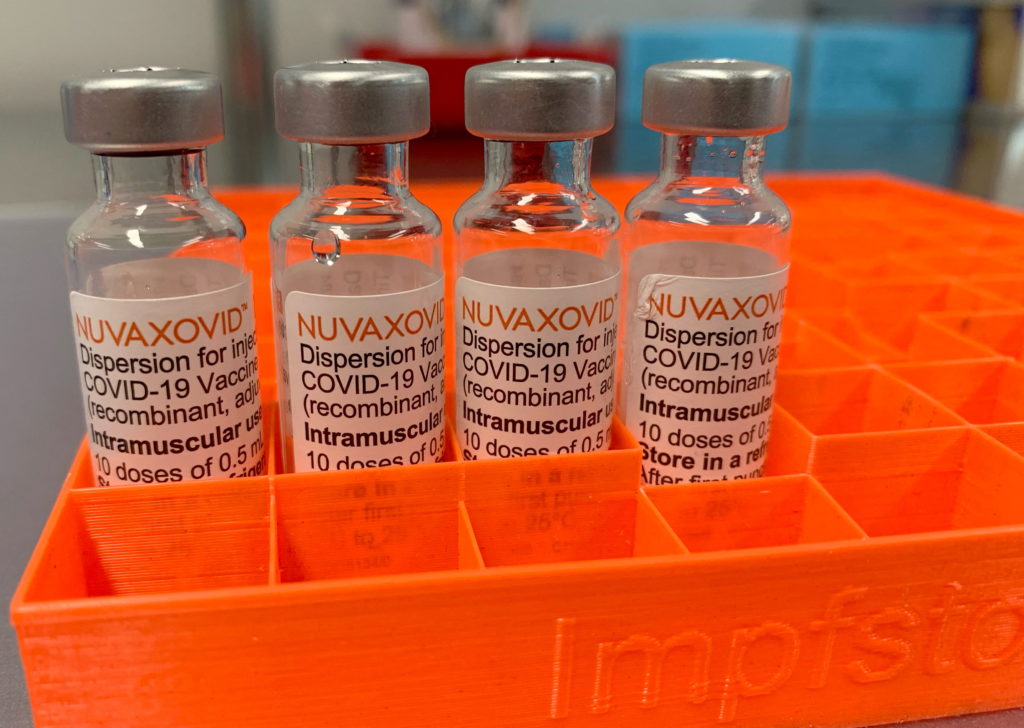
The FDA has finally granted approval to Novavax's COVID-19 vaccine, Nuvaxovid, but the approval comes with a significant caveat: unusual and arguably restrictive usage guidelines. This development, while welcomed by some, has left others questioning the vaccine's practical impact and future role in the ongoing pandemic response.
The approval, announced [Insert Date of Announcement], marks a significant milestone for Novavax, which has been striving for regulatory clearance for its protein-subunit vaccine for over two years. Unlike mRNA vaccines like Pfizer-BioNTech and Moderna, Nuvaxovid uses a more traditional technology, making it potentially attractive to vaccine-hesitant individuals who might be wary of the newer technologies. However, the FDA's decision to approve the vaccine only for individuals 18 years and older, and only as a two-dose primary series, raises several eyebrows.
Why the Restricted Usage?
The FDA's justification for the limited authorization remains unclear. While the agency has cited the vaccine's efficacy data, concerns remain about its performance compared to already authorized vaccines. Some experts suggest the restricted authorization reflects a lower perceived need for a new vaccine, given the widespread availability and success of existing options. The lack of authorization as a booster dose is particularly notable, limiting its potential use in the ongoing fight against evolving COVID-19 variants.
Several questions remain unanswered:
- Efficacy Concerns: While Nuvaxovid demonstrated efficacy in clinical trials, the data may not be as robust as that for other authorized vaccines. Further research is needed to fully understand its long-term effectiveness and protection against emerging variants.
- Market Competition: The existing vaccine landscape is already saturated. With high vaccination rates in many countries and readily available booster shots, the market demand for a new vaccine with restricted usage might be limited.
- Logistical Challenges: Rolling out a new vaccine with specific usage restrictions adds complexity to distribution and vaccination campaigns. Healthcare providers will need to navigate these guidelines, potentially leading to confusion and decreased uptake.
What This Means for the Future of COVID-19 Vaccination
The FDA's decision highlights the complexities of vaccine development and approval. While access to diverse vaccine platforms is crucial for a comprehensive pandemic response, the unusual restrictions placed on Nuvaxovid raise questions about its practical utility. The limited authorization may restrict its market penetration and limit its potential contribution to global vaccination efforts.
This decision also underscores the ongoing evolution of the pandemic and the need for continuous monitoring and adaptation of vaccination strategies. Further research and data on Nuvaxovid's long-term effectiveness and potential use as a booster are needed before a clearer assessment of its role can be made.
Looking Ahead: The future of Novavax's COVID-19 vaccine remains uncertain. Further clinical trials and data may influence future FDA decisions regarding broader authorization, including use as a booster. The current limitations, however, suggest that Nuvaxovid might not become the widespread game-changer initially anticipated. Only time will tell if it will find a significant niche in the global vaccination landscape.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, vaccine restrictions, protein-subunit vaccine, mRNA vaccine, vaccine efficacy, booster dose, pandemic response, vaccination campaign, global vaccination
(Note: Remember to replace "[Insert Date of Announcement]" with the actual date of the FDA's announcement.)

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax Vaccine Gets FDA Nod, But Faces Unusual Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Brexit Betrayal Claims Rock Eu Talks As Deadline Looms
May 20, 2025
Brexit Betrayal Claims Rock Eu Talks As Deadline Looms
May 20, 2025 -
 Copyright Battle Erupts Alison Brie And Dave Francos Together Accused Of Blatant Rip Off
May 20, 2025
Copyright Battle Erupts Alison Brie And Dave Francos Together Accused Of Blatant Rip Off
May 20, 2025 -
 America Y Sus Finales El Enfrentamiento Crucial Con Antonio Mohamed
May 20, 2025
America Y Sus Finales El Enfrentamiento Crucial Con Antonio Mohamed
May 20, 2025 -
 Helldivers 2 Masters Of Ceremony Warbond Drop Details For May 15th
May 20, 2025
Helldivers 2 Masters Of Ceremony Warbond Drop Details For May 15th
May 20, 2025 -
 Late Game Heroics Power Mud Hens Past Rail Riders
May 20, 2025
Late Game Heroics Power Mud Hens Past Rail Riders
May 20, 2025
Latest Posts
-
 Cnn Reports Israeli Strikes Hit Only Remaining Hospital In North Gaza
May 20, 2025
Cnn Reports Israeli Strikes Hit Only Remaining Hospital In North Gaza
May 20, 2025 -
 Eu Uk Brexit Talks A Delicate Balance On The Brink Of Collapse
May 20, 2025
Eu Uk Brexit Talks A Delicate Balance On The Brink Of Collapse
May 20, 2025 -
 The Real Story Behind Jamie Lee Curtis And Lindsay Lohans Bond
May 20, 2025
The Real Story Behind Jamie Lee Curtis And Lindsay Lohans Bond
May 20, 2025 -
 Peaky Blinders Creator Reveals Plans For A New Series And A Significant Shift
May 20, 2025
Peaky Blinders Creator Reveals Plans For A New Series And A Significant Shift
May 20, 2025 -
 Bold Bets Drive 5 B Influx Into Bitcoin Etfs Market Analysis And Predictions
May 20, 2025
Bold Bets Drive 5 B Influx Into Bitcoin Etfs Market Analysis And Predictions
May 20, 2025
