Novavax Vaccine Gets FDA Nod, But With Uncommon Usage Constraints
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
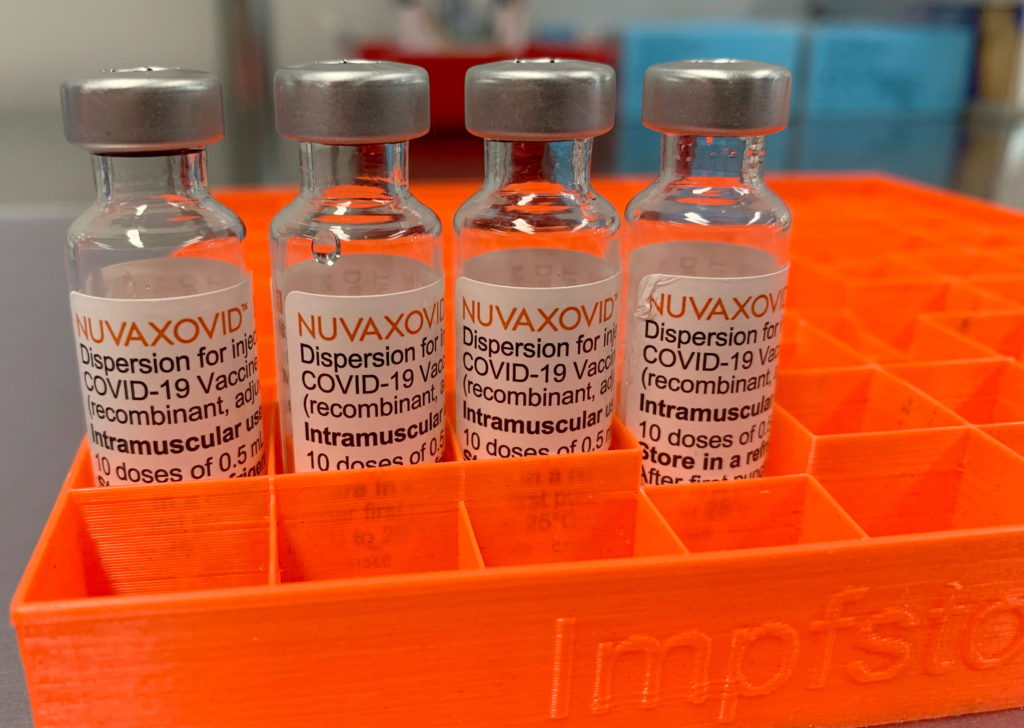
Novavax Vaccine Gets FDA Nod, but with Uncommon Usage Constraints
The FDA has finally granted full approval to the Novavax COVID-19 vaccine, Nuvaxovid, but with significant caveats that limit its widespread use. This decision, while seemingly a victory for Novavax, comes with considerable constraints that raise questions about the vaccine's practical impact on the ongoing pandemic response. The approval, announced on [Insert Date of Announcement], marks a significant milestone for the company, but the accompanying limitations are drawing considerable scrutiny from public health experts and the medical community.
Limited Applicability: A Key Restriction
The FDA's approval is not a blanket endorsement. The agency has approved Nuvaxovid only for individuals aged 18 years and older. This is noteworthy, as many other widely used COVID-19 vaccines have received authorization for younger age groups. Furthermore, the approval is specifically not for use as a primary vaccination series for individuals who have already received another COVID-19 vaccine. This significantly restricts the potential user base, making its role in the current vaccination landscape unclear.
Why the Limitations? Understanding the FDA's Decision
The FDA's decision to approve Nuvaxovid with such limitations likely stems from several factors. While the vaccine demonstrated efficacy in clinical trials, the data might not have been as robust or extensive as that supporting other authorized vaccines. Additionally, the emergence of new COVID-19 variants and the widespread availability of other vaccines could have influenced the agency's cautious approach. The limited data on the vaccine's performance against newer variants might also have played a role in restricting its broader application.
The Novavax Technology: A Different Approach
Nuvaxovid employs a protein subunit technology, a more traditional approach compared to the mRNA technology used in vaccines from Pfizer-BioNTech and Moderna. This protein-based technology has been used in other vaccines for decades, and some believe it might offer advantages in terms of safety and public perception for vaccine-hesitant individuals. However, the manufacturing process can be more complex and slower than mRNA production, potentially contributing to the delayed approval and limited availability.
Future Prospects: What Does This Mean for Novavax and COVID-19 Vaccination?
The FDA's approval, albeit with limitations, provides Novavax with a crucial foothold in the COVID-19 vaccine market. However, the narrow application significantly impacts its potential market share. The vaccine's role might be limited to specific populations or situations where other vaccines are contraindicated. Further research and data on the vaccine's effectiveness against emerging variants are crucial to expanding its usage and determining its long-term significance in the fight against COVID-19.
Looking Ahead: The Need for Ongoing Monitoring
The ongoing monitoring of the vaccine's safety and efficacy will be vital. Post-market surveillance will help to assess its real-world performance and identify any potential issues. This data will be instrumental in informing future decisions about the vaccine's role in vaccination strategies and potential expansion of its usage.
Keywords: Novavax, COVID-19 vaccine, Nuvaxovid, FDA approval, vaccine limitations, protein subunit vaccine, mRNA vaccine, COVID-19 vaccination, public health, vaccine safety, vaccine efficacy.
Call to Action (subtle): Stay informed about the latest developments in COVID-19 vaccination by following reputable sources such as the CDC and FDA websites. Consult your healthcare provider for personalized advice on COVID-19 vaccination.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax Vaccine Gets FDA Nod, But With Uncommon Usage Constraints. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Jamie Lee Curtis Opens Up About Staying Connected With Lindsay Lohan
May 20, 2025
Jamie Lee Curtis Opens Up About Staying Connected With Lindsay Lohan
May 20, 2025 -
 Jon Jones Ufc Kept Aspinalls Injury A Secret From Fans
May 20, 2025
Jon Jones Ufc Kept Aspinalls Injury A Secret From Fans
May 20, 2025 -
 Bali Seeks Global Help To Improve Tourist Conduct
May 20, 2025
Bali Seeks Global Help To Improve Tourist Conduct
May 20, 2025 -
 Jenn Sterger On Brett Favre Sext Scandal I Was Never Treated Like A Person
May 20, 2025
Jenn Sterger On Brett Favre Sext Scandal I Was Never Treated Like A Person
May 20, 2025 -
 Critically Acclaimed Wwi Film With Daniel Craig Cillian Murphy And Tom Hardy Available To Stream
May 20, 2025
Critically Acclaimed Wwi Film With Daniel Craig Cillian Murphy And Tom Hardy Available To Stream
May 20, 2025
Latest Posts
-
 Over 5 Billion Inflows Bitcoin Etf Investment Surges
May 21, 2025
Over 5 Billion Inflows Bitcoin Etf Investment Surges
May 21, 2025 -
 New Guidelines Aim To Improve Tourist Conduct In Bali
May 21, 2025
New Guidelines Aim To Improve Tourist Conduct In Bali
May 21, 2025 -
 Joe Bidens Prostate Cancer What We Know And What It Means
May 21, 2025
Joe Bidens Prostate Cancer What We Know And What It Means
May 21, 2025 -
 New Clues Emerge From A Canadian Pachyrhinosaurus Fossil Bed
May 21, 2025
New Clues Emerge From A Canadian Pachyrhinosaurus Fossil Bed
May 21, 2025 -
 Record Ratings Snl Concludes 50th Season On A High Note
May 21, 2025
Record Ratings Snl Concludes 50th Season On A High Note
May 21, 2025
