Conditional FDA Approval: Novavax COVID-19 Vaccine Faces Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Conditional FDA Approval: Novavax COVID-19 Vaccine Faces Usage Restrictions
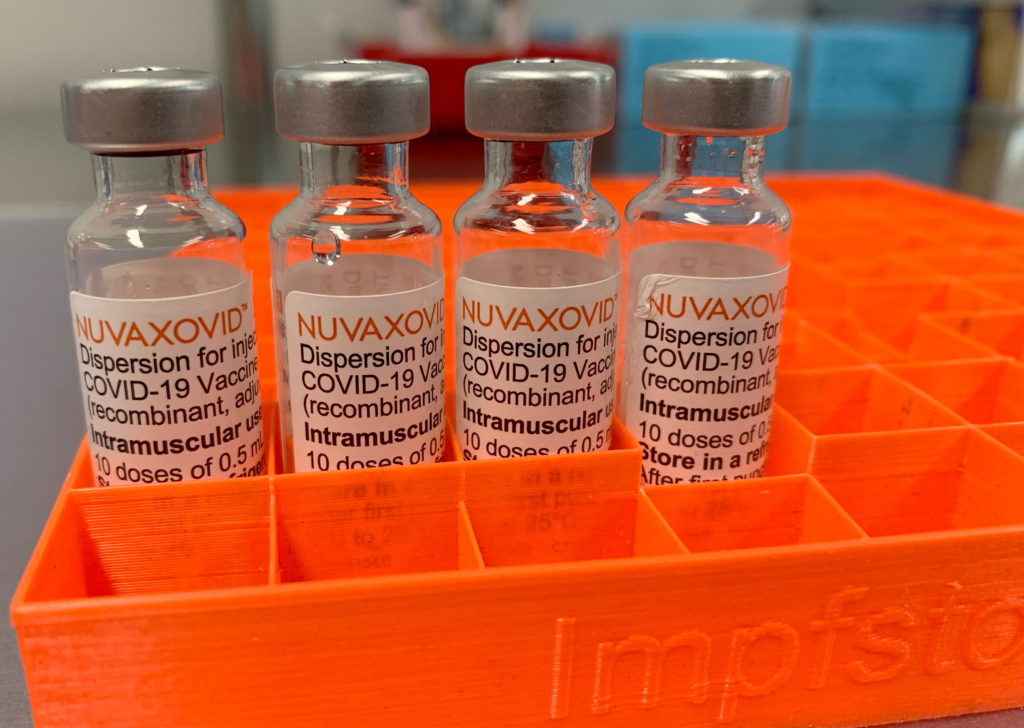
The Novavax COVID-19 vaccine, Nuvaxovid, has received conditional FDA approval, marking a significant step for the pharmaceutical company. However, this approval comes with considerable usage restrictions, impacting its potential market share and raising questions about its future role in the ongoing pandemic. This article delves into the specifics of the FDA's decision, exploring the reasons behind the limitations and analyzing their implications.
Limited Authorization and Specific Target Group
The FDA's conditional approval is not a blanket authorization for widespread use. Instead, Nuvaxovid is authorized for use in individuals 18 years of age and older. This age restriction contrasts with other widely available COVID-19 vaccines which have broader age ranges, including authorization for adolescents and even younger children in some cases. This limitation significantly narrows the potential user base for Novavax's vaccine.
Furthermore, the FDA's approval is contingent on ongoing monitoring of the vaccine's safety and effectiveness. This means that the conditional approval could be revoked or modified based on future data analysis. Such ongoing surveillance is standard practice for newly approved vaccines, but it highlights the uncertainties surrounding Nuvaxovid's long-term efficacy and safety profile.
Why the Restrictions? Concerns and Considerations
The FDA's decision to impose these restrictions likely stems from several factors:
- Comparative Efficacy: Data suggests that Nuvaxovid's efficacy might be slightly lower compared to other leading COVID-19 vaccines currently available. While it still offers substantial protection against severe illness, hospitalization, and death, the smaller margin could be a factor in the limited authorization.
- Limited Trial Data: The clinical trials supporting the FDA's approval might have limitations in terms of sample size or representation of diverse populations. Further research and data collection are needed to solidify its efficacy and safety profile across different demographic groups.
- Emergence of Variants: The ever-evolving nature of the SARS-CoV-2 virus, with the emergence of new variants, poses a continuous challenge for vaccine efficacy. The FDA's cautious approach might reflect concerns about Nuvaxovid's ability to effectively combat emerging variants.
Impact on Vaccine Landscape and Public Health
The conditional approval and subsequent limitations raise questions about Nuvaxovid's impact on the existing COVID-19 vaccine landscape. While offering an alternative for individuals who might have concerns about mRNA vaccines, the restricted usage will likely limit its overall impact on public health initiatives aiming for broad vaccination coverage.
Moving Forward: Future Prospects for Novavax
Novavax will need to continue monitoring and reporting data to the FDA to maintain its conditional approval. Further clinical trials and research are crucial to expand the authorization to other age groups and address concerns regarding efficacy against emerging variants. The company's future success will depend on its ability to overcome these limitations and demonstrate the long-term benefits and safety of Nuvaxovid.
Keywords: Novavax, COVID-19 vaccine, Nuvaxovid, FDA approval, conditional approval, vaccine restrictions, vaccine efficacy, vaccine safety, coronavirus, pandemic, public health, mRNA vaccines
Related Articles: (Links to relevant articles on other COVID-19 vaccines or FDA approvals would go here)
Disclaimer: This article provides information for educational purposes only and is not intended as medical advice. Always consult with a healthcare professional for any health concerns or before making any decisions related to your health or treatment.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval: Novavax COVID-19 Vaccine Faces Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 The Unexpected Friendship Jamie Lee Curtis On Her Connection With Lindsay Lohan
May 20, 2025
The Unexpected Friendship Jamie Lee Curtis On Her Connection With Lindsay Lohan
May 20, 2025 -
 Us Treasury Market Reacts Feds 2025 Rate Cut Projection
May 20, 2025
Us Treasury Market Reacts Feds 2025 Rate Cut Projection
May 20, 2025 -
 Bbc And Gary Lineker Part Ways The Match Of The Day Fallout
May 20, 2025
Bbc And Gary Lineker Part Ways The Match Of The Day Fallout
May 20, 2025 -
 Colombian Model And Mexican Influencer Killed A Grim Reminder Of The Femicide Crisis
May 20, 2025
Colombian Model And Mexican Influencer Killed A Grim Reminder Of The Femicide Crisis
May 20, 2025 -
 Eu Uk Brexit Talks Last Minute Scramble Amidst Accusations Of Betrayal
May 20, 2025
Eu Uk Brexit Talks Last Minute Scramble Amidst Accusations Of Betrayal
May 20, 2025
Latest Posts
-
 Train Tragedy Two Adults Dead Children Injured After Family Struck On Bridge
May 20, 2025
Train Tragedy Two Adults Dead Children Injured After Family Struck On Bridge
May 20, 2025 -
 The Death Of The Herd Understanding The Pachyrhinosaurus Fossil Site In Canada
May 20, 2025
The Death Of The Herd Understanding The Pachyrhinosaurus Fossil Site In Canada
May 20, 2025 -
 Brexit Betrayal Eu Leaders Accuse Uk In Final Stages Of Negotiations
May 20, 2025
Brexit Betrayal Eu Leaders Accuse Uk In Final Stages Of Negotiations
May 20, 2025 -
 A Candid Conversation Jamie Lee Curtis Reflects On Her Relationship With Lindsay Lohan
May 20, 2025
A Candid Conversation Jamie Lee Curtis Reflects On Her Relationship With Lindsay Lohan
May 20, 2025 -
 May 15th Brings New Warbond Drops To Helldivers 2s Masters Of Ceremony
May 20, 2025
May 15th Brings New Warbond Drops To Helldivers 2s Masters Of Ceremony
May 20, 2025
