Conditional FDA Approval: Novavax COVID-19 Vaccine's Limited Use
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
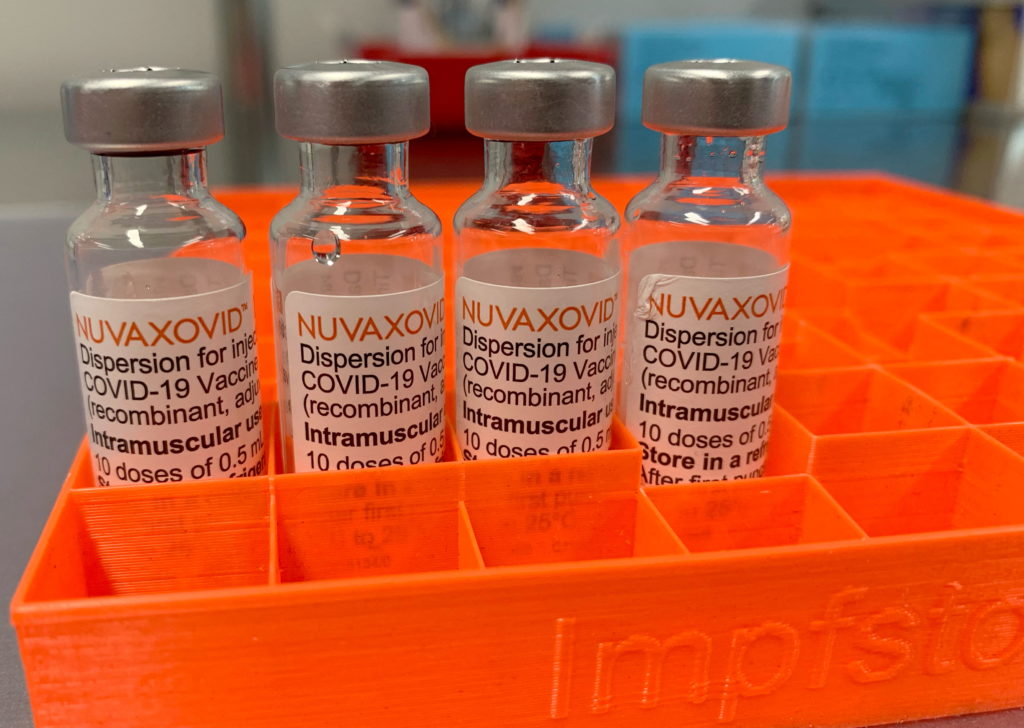
Conditional FDA Approval: Novavax COVID-19 Vaccine's Limited Use
The FDA's conditional approval of the Novavax COVID-19 vaccine, NVX-CoV2373, marks a significant yet nuanced development in the ongoing fight against the pandemic. While hailed as a welcome addition to the vaccine arsenal, its limited use and specific target audience raise important questions about its overall impact and accessibility.
A New Contender in the COVID-19 Vaccine Landscape
The Novavax vaccine, utilizing a protein subunit technology distinct from mRNA vaccines like Pfizer-BioNTech and Moderna, offers a different approach to COVID-19 immunization. This protein-based technology has been used in other vaccines for decades, potentially appealing to individuals hesitant about mRNA technology. The FDA's approval, however, comes with caveats that significantly restrict its immediate widespread adoption.
Limited Use: Who Can Get the Novavax Vaccine?
The FDA's authorization is currently limited to individuals aged 18 years and older. This excludes children and adolescents, a demographic crucial for achieving herd immunity. Furthermore, the vaccine's approval is conditional, meaning ongoing monitoring of its safety and effectiveness is required. This conditional approval differs from a full approval, indicating a more cautious approach by regulatory authorities.
Why the Limited Rollout?
Several factors contribute to the Novavax vaccine's limited rollout. Firstly, the relatively late arrival of the vaccine to the market means it faces competition from already established and widely distributed alternatives. Secondly, production capacity may be a limiting factor, influencing the immediate availability of the vaccine. Finally, the conditional approval itself reflects a need for further data collection and ongoing safety monitoring.
Potential Advantages and Disadvantages
-
Advantages: The protein subunit technology may appeal to individuals hesitant about mRNA vaccines. Its different mechanism of action might also prove beneficial in specific scenarios or for individuals who experienced adverse effects from other vaccines.
-
Disadvantages: The limited age range and conditional approval restrict its immediate impact. The late arrival to market, combined with potential production constraints, might limit accessibility.
The Future of the Novavax Vaccine
The future success of the Novavax vaccine hinges on several factors: its ability to demonstrate long-term safety and effectiveness in clinical trials, the expansion of its authorized age range, and increased production capacity to meet demand. The FDA's conditional approval represents a cautious yet promising step, but its widespread impact remains to be seen. Further research and monitoring are crucial to fully assess the vaccine’s long-term benefits and potential role in global vaccination efforts. The availability and accessibility of this vaccine will be a key indicator of its overall success in combating COVID-19.
Call to Action: Stay informed about the latest updates on COVID-19 vaccines and consult with your healthcare provider to determine which vaccine is most appropriate for you. Visit the for up-to-date information on COVID-19 vaccination.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval: Novavax COVID-19 Vaccine's Limited Use. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 New Guidelines Aim To Improve Tourist Conduct In Bali
May 21, 2025
New Guidelines Aim To Improve Tourist Conduct In Bali
May 21, 2025 -
 Helldivers 2 May 15th Update Master Of Ceremony Warbond Drop Details
May 21, 2025
Helldivers 2 May 15th Update Master Of Ceremony Warbond Drop Details
May 21, 2025 -
 Watch Now A Powerful Wwi Drama Featuring Daniel Craig Cillian Murphy And Tom Hardy
May 21, 2025
Watch Now A Powerful Wwi Drama Featuring Daniel Craig Cillian Murphy And Tom Hardy
May 21, 2025 -
 Did The Ufc Withhold Key Information On Tom Aspinall Jones Demands Answers
May 21, 2025
Did The Ufc Withhold Key Information On Tom Aspinall Jones Demands Answers
May 21, 2025 -
 Putin Shows Trumps Diminished Global Leverage
May 21, 2025
Putin Shows Trumps Diminished Global Leverage
May 21, 2025
Latest Posts
-
 Market Rally Continues S And P 500s Six Day Winning Streak And Positive Market Sentiment
May 21, 2025
Market Rally Continues S And P 500s Six Day Winning Streak And Positive Market Sentiment
May 21, 2025 -
 St Louis Tornado Community Rallies To Rebuild After Devastating Storm
May 21, 2025
St Louis Tornado Community Rallies To Rebuild After Devastating Storm
May 21, 2025 -
 Jon Jones Vs Tom Aspinall Analyzing The Controversy Behind Jones Latest Statement
May 21, 2025
Jon Jones Vs Tom Aspinall Analyzing The Controversy Behind Jones Latest Statement
May 21, 2025 -
 Weaning Your Child Off Pacifiers And Thumb Sucking Age Methods And Challenges
May 21, 2025
Weaning Your Child Off Pacifiers And Thumb Sucking Age Methods And Challenges
May 21, 2025 -
 Bali Tourism Overhaul New Regulations Target Unacceptable Tourist Conduct
May 21, 2025
Bali Tourism Overhaul New Regulations Target Unacceptable Tourist Conduct
May 21, 2025
