FDA Approval For Novavax COVID-19 Vaccine: Strict Usage Guidelines Announced
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Approval for Novavax COVID-19 Vaccine: Strict Usage Guidelines Announced
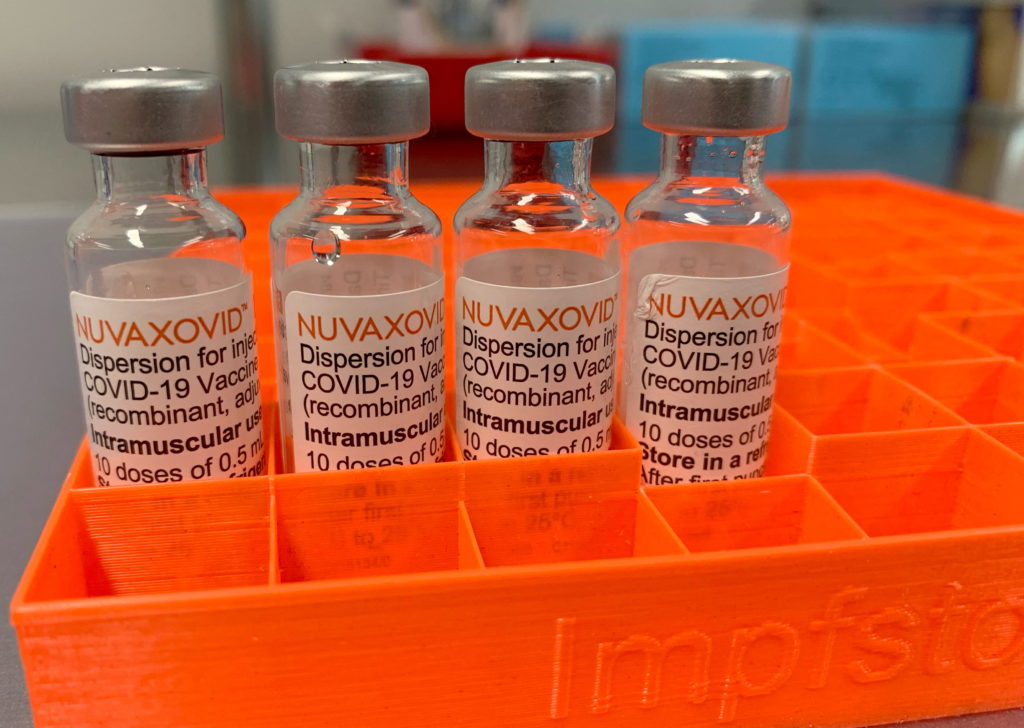
Novavax's COVID-19 vaccine, Nuvaxovid, finally receives FDA approval, but with caveats. The long-awaited approval from the Food and Drug Administration (FDA) marks a significant step forward in the fight against COVID-19, offering an alternative for those hesitant about mRNA vaccines. However, the FDA's announcement also includes strict usage guidelines, impacting its widespread rollout and accessibility.
The approval, granted under the agency's Emergency Use Authorization (EUA) pathway, is a welcome development for public health officials hoping to boost vaccination rates. Unlike the Pfizer-BioNTech and Moderna vaccines which utilize mRNA technology, Nuvaxovid uses a more traditional protein subunit approach. This difference has led many to eagerly anticipate its arrival, hoping it would address vaccine hesitancy stemming from concerns over mRNA technology.
Understanding the Nuvaxovid Approval and its Limitations
The FDA's approval is specifically for individuals 18 years of age and older. Crucially, the agency emphasizes that Nuvaxovid is not authorized for use as a booster shot. This limits its immediate impact, particularly as booster campaigns continue to be a cornerstone of COVID-19 mitigation strategies. The strict age restriction also means that younger populations will continue to rely on other approved vaccines.
The FDA's decision comes after a thorough review of clinical trial data demonstrating Nuvaxovid's efficacy and safety profile. However, the agency highlights the need for careful monitoring of potential side effects, leading to the implementation of robust surveillance protocols. This underscores the cautious approach adopted by the FDA in granting approval, emphasizing the importance of continued safety monitoring.
Strict Usage Guidelines: What They Mean for Patients
The strict usage guidelines are designed to ensure the safe and effective deployment of Nuvaxovid. These guidelines include:
- Age Restriction: Only individuals 18 years and older are currently eligible to receive the vaccine.
- Dosage: The vaccine requires a two-dose primary vaccination series, administered three weeks apart.
- Booster Shot Exclusion: At present, Nuvaxovid is not approved for use as a booster dose. Further research will determine its suitability for booster applications.
- Monitoring for Adverse Events: Healthcare providers are mandated to carefully monitor patients for potential side effects following vaccination.
The FDA's emphasis on monitoring highlights the need for ongoing vigilance. Healthcare professionals are urged to report any serious adverse events to the Vaccine Adverse Event Reporting System (VAERS). This proactive approach underscores the FDA's commitment to ensuring patient safety.
Implications for the Future of COVID-19 Vaccination
The FDA approval of Nuvaxovid represents a crucial step in diversifying the available COVID-19 vaccines. The availability of a protein subunit vaccine offers a different approach for those who have been hesitant about mRNA technology. However, the limited availability and the strict usage guidelines mean that it won’t immediately solve the challenges of vaccine access and hesitancy.
Further research is needed to fully explore the potential of Nuvaxovid as a booster shot and for use in younger populations. This ongoing research will be crucial in maximizing the vaccine's potential contribution to the ongoing fight against COVID-19. The FDA's decision underscores the importance of data-driven decisions in vaccine approvals, prioritizing both efficacy and safety. The future impact of Nuvaxovid remains to be seen, but its arrival offers a new avenue in the global effort to combat the pandemic.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein subunit vaccine, mRNA vaccine, vaccine hesitancy, vaccination, usage guidelines, booster shot, safety, efficacy, clinical trials, VAERS, public health.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine: Strict Usage Guidelines Announced. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Jamie Lee Curtis Defends Lindsay Lohan A History Of Honest Friendship
May 20, 2025
Jamie Lee Curtis Defends Lindsay Lohan A History Of Honest Friendship
May 20, 2025 -
 Over 5 Billion Invested In Bitcoin Etfs Analyzing The Bold Market Bets
May 20, 2025
Over 5 Billion Invested In Bitcoin Etfs Analyzing The Bold Market Bets
May 20, 2025 -
 Untold A J Perez Details Threats From Brett Favres Camp
May 20, 2025
Untold A J Perez Details Threats From Brett Favres Camp
May 20, 2025 -
 Behind The Scenes Of Fall Of Favre A Directors Perspective On The Complex Legacy Of Brett Favre
May 20, 2025
Behind The Scenes Of Fall Of Favre A Directors Perspective On The Complex Legacy Of Brett Favre
May 20, 2025 -
 Super League Coaching Rivalry Arthur Secures Victory
May 20, 2025
Super League Coaching Rivalry Arthur Secures Victory
May 20, 2025
Latest Posts
-
 Get The Master Of Ceremony Warbonds In Helldivers 2 On May 15th
May 20, 2025
Get The Master Of Ceremony Warbonds In Helldivers 2 On May 15th
May 20, 2025 -
 From Destruction To Recovery St Louis After A Devastating Tornado
May 20, 2025
From Destruction To Recovery St Louis After A Devastating Tornado
May 20, 2025 -
 Lufthansa Co Pilot Collapses Flight Continues Pilotless For 10 Minutes Investigation Reveals
May 20, 2025
Lufthansa Co Pilot Collapses Flight Continues Pilotless For 10 Minutes Investigation Reveals
May 20, 2025 -
 Slow Burn Brilliance Why The Last Of Uss Quiet Moments Resonate
May 20, 2025
Slow Burn Brilliance Why The Last Of Uss Quiet Moments Resonate
May 20, 2025 -
 Three Decades Of Service Reflecting On Alito And Roberts Supreme Court Careers
May 20, 2025
Three Decades Of Service Reflecting On Alito And Roberts Supreme Court Careers
May 20, 2025
