FDA Approves Novavax COVID-19 Vaccine: Usage Restrictions Explained
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Approves Novavax COVID-19 Vaccine: Usage Restrictions Explained
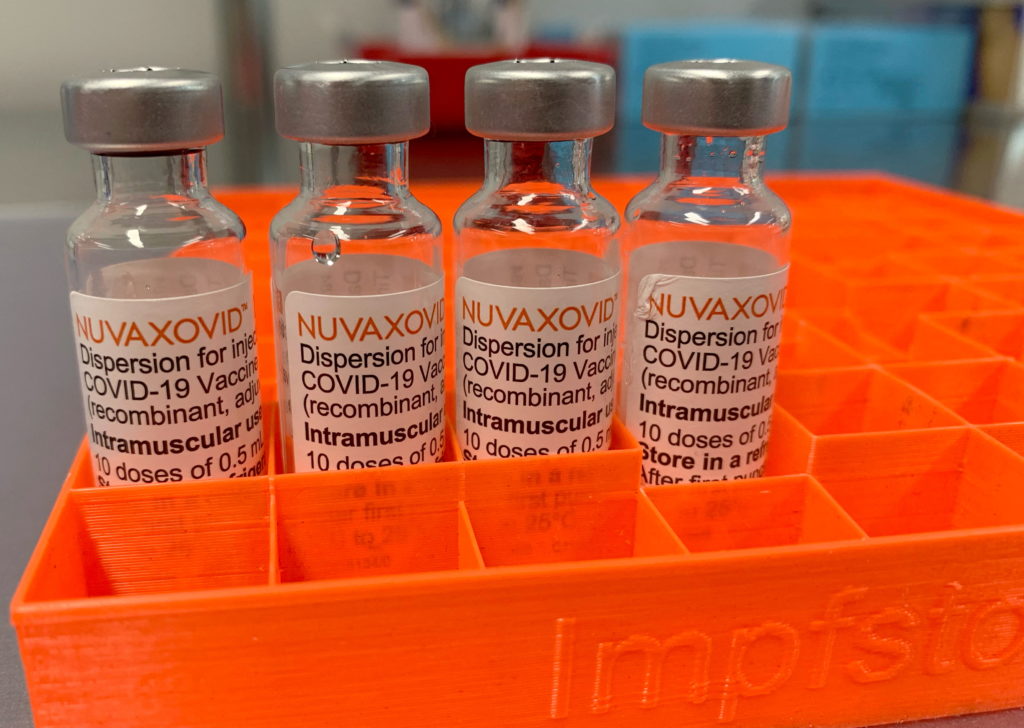
The FDA's approval of the Novavax COVID-19 vaccine marks a significant step in the ongoing fight against the pandemic. This protein-subunit vaccine offers a different approach compared to the mRNA vaccines from Pfizer-BioNTech and Moderna, potentially appealing to individuals hesitant about the newer technologies. However, understanding the specific usage restrictions is crucial for healthcare providers and the public alike.
Understanding the Novavax Vaccine:
Unlike mRNA vaccines, which use messenger RNA to instruct cells to produce a viral protein, the Novavax vaccine utilizes a more traditional method. It contains purified, inactive pieces of the SARS-CoV-2 virus (the spike protein) that trigger an immune response without causing illness. This approach has been used in vaccines for other diseases for decades, potentially increasing acceptance among vaccine-hesitant individuals. This makes it a valuable addition to the arsenal of COVID-19 vaccines.
FDA Approval and Authorization:
The FDA granted Novavax's COVID-19 vaccine, marketed as Nuvaxovid, full approval for individuals 18 years of age and older. This contrasts with the initial Emergency Use Authorizations (EUAs) granted to other COVID-19 vaccines. Full approval signifies a rigorous review process, solidifying its safety and efficacy profile.
Key Usage Restrictions:
While a welcome addition, the Novavax vaccine does come with specific usage restrictions:
- Age Restriction: Currently, the FDA approval is only for individuals aged 18 and older. Further studies are needed to determine its safety and efficacy in younger populations.
- Two-Dose Primary Series: The vaccine requires a two-dose primary series administered three weeks apart. This is standard for many traditional vaccines.
- Allergic Reactions: As with all vaccines, individuals with a known history of severe allergic reactions to any component of the Novavax vaccine should avoid it. Healthcare providers should be aware of potential allergic reactions and have appropriate treatment readily available. This includes monitoring for anaphylaxis, a severe allergic reaction.
- Pre-existing Conditions: While generally safe, individuals with specific pre-existing conditions should consult their physician before receiving the vaccination. This is standard practice for any vaccination.
- Pregnancy and Breastfeeding: Data on pregnant and breastfeeding individuals is still being gathered. Consult with a healthcare professional to weigh the benefits and risks in these populations.
Comparing Novavax to Other COVID-19 Vaccines:
The Novavax vaccine offers a distinct alternative to the widely used mRNA vaccines. Its protein-subunit technology might appeal to individuals concerned about mRNA technology's novelty. However, it's important to remember that all currently authorized COVID-19 vaccines have proven highly effective at preventing severe illness, hospitalization, and death. The choice of vaccine should be made in consultation with a healthcare provider based on individual circumstances and preferences. You can learn more about other COVID-19 vaccines on the .
Looking Ahead:
The FDA's approval of the Novavax COVID-19 vaccine broadens the options available to combat the virus. Understanding the usage restrictions is vital for safe and effective deployment. As always, consult your physician to determine the best vaccination strategy for your individual needs. Staying informed about COVID-19 and vaccination updates is crucial for public health. Check the regularly for the latest information.
Keywords: Novavax, COVID-19 vaccine, FDA approval, vaccine usage restrictions, protein-subunit vaccine, mRNA vaccine, Nuvaxovid, vaccine safety, vaccine efficacy, allergic reactions, vaccination, COVID-19, pandemic, public health.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approves Novavax COVID-19 Vaccine: Usage Restrictions Explained. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 News Analysis A Deep Dive Into The Surrender Summit And Post Office Choir Stories
May 20, 2025
News Analysis A Deep Dive Into The Surrender Summit And Post Office Choir Stories
May 20, 2025 -
 Toledo Mud Hens Rout Erie With 20 Run Outburst Mendoza Lee Lead The Charge
May 20, 2025
Toledo Mud Hens Rout Erie With 20 Run Outburst Mendoza Lee Lead The Charge
May 20, 2025 -
 Bitcoin Etf Investment 5 Billion Influx And Future Market Outlook
May 20, 2025
Bitcoin Etf Investment 5 Billion Influx And Future Market Outlook
May 20, 2025 -
 Jjs Wasted Love An Austrian Eurovision 2025 Victory
May 20, 2025
Jjs Wasted Love An Austrian Eurovision 2025 Victory
May 20, 2025 -
 Ufc Accused Of Concealing Aspinall Information Jon Jones Speaks Out
May 20, 2025
Ufc Accused Of Concealing Aspinall Information Jon Jones Speaks Out
May 20, 2025
Latest Posts
-
 10 Minutes Without A Pilot Lufthansa Flight Incident Report Highlights Co Pilots Collapse
May 20, 2025
10 Minutes Without A Pilot Lufthansa Flight Incident Report Highlights Co Pilots Collapse
May 20, 2025 -
 Femicide Causes Consequences And Strategies For Prevention
May 20, 2025
Femicide Causes Consequences And Strategies For Prevention
May 20, 2025 -
 Dehumanized Jenn Sterger Recounts Her Experience In The Brett Favre Scandal
May 20, 2025
Dehumanized Jenn Sterger Recounts Her Experience In The Brett Favre Scandal
May 20, 2025 -
 Supreme Court Justices Alito And Roberts A Legacy In The Making
May 20, 2025
Supreme Court Justices Alito And Roberts A Legacy In The Making
May 20, 2025 -
 Understanding Femicide Data Trends And The Fight For Justice
May 20, 2025
Understanding Femicide Data Trends And The Fight For Justice
May 20, 2025
