Limited FDA Approval For Novavax COVID-19 Vaccine: Understanding The Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Limited FDA Approval for Novavax COVID-19 Vaccine: Understanding the Restrictions
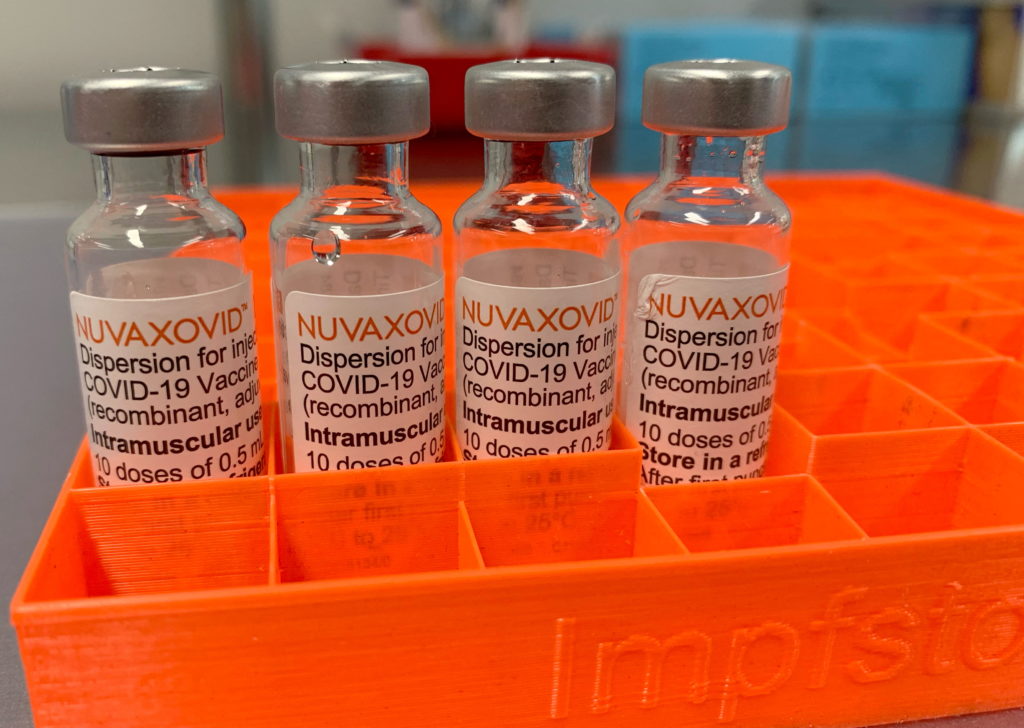
The Novavax COVID-19 vaccine, Nuvaxovid, has received limited approval from the FDA, raising questions about its availability and use. While this marks a significant development in the fight against the pandemic, it's crucial to understand the nuances of this approval and its implications. This article breaks down the FDA's decision, addressing key concerns and clarifying the restrictions surrounding the vaccine's use.
A Step Forward, But With Cautions:
The FDA's authorization is not a full approval like that granted to Pfizer-BioNTech and Moderna vaccines. Instead, it's an Emergency Use Authorization (EUA), granted under the urgency of the ongoing pandemic. This distinction is important because it reflects the fact that while the vaccine has proven safe and effective, long-term data is still being collected. The EUA allows for quicker deployment but comes with ongoing monitoring and potential revisions.
What the FDA Approval Means:
The EUA allows the Novavax vaccine to be administered to individuals 18 years of age and older. The decision followed rigorous testing, demonstrating the vaccine's efficacy in preventing COVID-19. However, the limited nature of the EUA needs emphasis.
Key Restrictions and Limitations:
- Age Restriction: Currently, the EUA only covers adults 18 and older. Trials for younger age groups are ongoing, and future approvals may expand the eligible population.
- Limited Data on Variants: While effective against earlier strains, further research is needed to fully assess its effectiveness against emerging variants of concern. The efficacy may vary depending on the circulating strain.
- Ongoing Monitoring: Like all vaccines under EUA, Novavax will continue to be monitored for safety and efficacy. Any adverse events or changes in effectiveness will be carefully evaluated.
- Supply and Availability: The rollout of the Novavax vaccine is likely to be phased, meaning widespread availability may take time depending on manufacturing capacity and distribution networks. Check with your local health authorities for the latest updates on vaccine availability in your area.
Why the Limited Approval?
The FDA's decision reflects a cautious approach prioritizing safety and thorough evaluation. The EUA allows for immediate use while ongoing studies provide more comprehensive long-term data. This strategy is common for vaccines under emergency situations. It allows for rapid response while maintaining a rigorous scientific standard.
Comparing Novavax to Other COVID-19 Vaccines:
Novavax uses a different technology than the mRNA vaccines from Pfizer-BioNTech and Moderna. It employs a protein subunit technology, a more traditional approach that may appeal to individuals hesitant about mRNA vaccines. However, it's crucial to remember that all authorized COVID-19 vaccines have demonstrated efficacy in preventing severe illness, hospitalization, and death.
The Road Ahead:
The FDA's limited approval of the Novavax COVID-19 vaccine is a significant development, offering an alternative option for individuals seeking vaccination. However, understanding the restrictions and ongoing monitoring is essential. Stay informed by checking reliable sources like the and the for the most up-to-date information. Consulting with your healthcare provider is recommended to determine the best vaccination strategy for you. Remember, vaccination remains a crucial step in protecting yourself and your community against COVID-19.
Keywords: Novavax, COVID-19 vaccine, FDA approval, EUA, Emergency Use Authorization, vaccine restrictions, Nuvaxovid, protein subunit vaccine, mRNA vaccine, vaccine safety, vaccine efficacy, COVID-19 variants, vaccine availability, CDC, FDA.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Limited FDA Approval For Novavax COVID-19 Vaccine: Understanding The Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Close Super League Battle Leeds Rhinos Defeat Hull Fc 18 16
May 20, 2025
Close Super League Battle Leeds Rhinos Defeat Hull Fc 18 16
May 20, 2025 -
 Jamie Lee Curtis Lindsay Lohan Has Always Kept It Real
May 20, 2025
Jamie Lee Curtis Lindsay Lohan Has Always Kept It Real
May 20, 2025 -
 Sundance Film Together Faces Copyright Infringement Claim Alison Brie And Dave Franco Involved
May 20, 2025
Sundance Film Together Faces Copyright Infringement Claim Alison Brie And Dave Franco Involved
May 20, 2025 -
 Conditional Fda Approval Novavax Covid 19 Vaccine Faces Usage Restrictions
May 20, 2025
Conditional Fda Approval Novavax Covid 19 Vaccine Faces Usage Restrictions
May 20, 2025 -
 Dutertes Local Election Triumph The Hagues Potential Impact
May 20, 2025
Dutertes Local Election Triumph The Hagues Potential Impact
May 20, 2025
Latest Posts
-
 Brett Favre Sexting Scandal Jenn Sterger Details The Emotional Fallout
May 20, 2025
Brett Favre Sexting Scandal Jenn Sterger Details The Emotional Fallout
May 20, 2025 -
 Peaky Blinders Steven Knight Announces New Series Details Significant Shift
May 20, 2025
Peaky Blinders Steven Knight Announces New Series Details Significant Shift
May 20, 2025 -
 What Is Femicide Examining The Problem And Potential Solutions
May 20, 2025
What Is Femicide Examining The Problem And Potential Solutions
May 20, 2025 -
 Jon Jones Strip The Duck Comment A Deeper Dive Into The Mma World
May 20, 2025
Jon Jones Strip The Duck Comment A Deeper Dive Into The Mma World
May 20, 2025 -
 Misbehaving Tourists Beware Bali Implements New Guidelines
May 20, 2025
Misbehaving Tourists Beware Bali Implements New Guidelines
May 20, 2025
