Novavax COVID-19 Vaccine Approved By FDA, But With Limitations
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Approved by FDA, But with Limitations
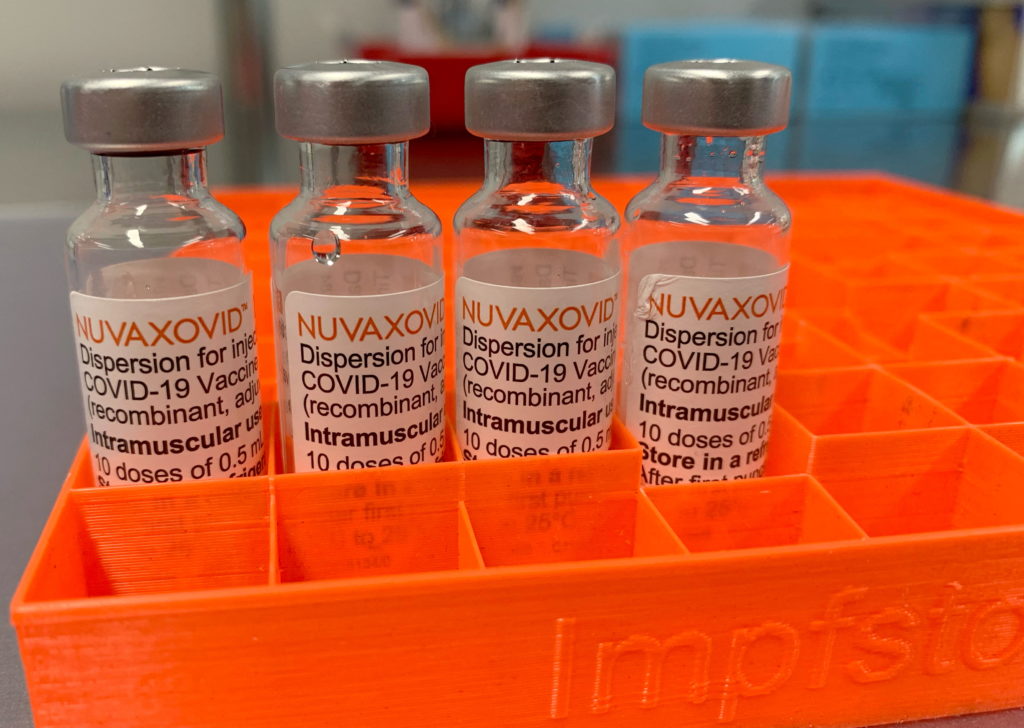
The FDA has finally granted full approval to the Novavax COVID-19 vaccine, but with significant caveats. This long-awaited approval offers a new option for those hesitant about mRNA vaccines, but its limited scope raises questions about its overall impact. The approval comes after months of review and follows the vaccine's emergency use authorization. Let's delve into the details.
What Does Full FDA Approval Mean for Novavax?
Full FDA approval signifies that the agency has conducted a thorough review of the Novavax vaccine's safety and effectiveness data, meeting their stringent standards. This differs significantly from the previous Emergency Use Authorization (EUA), which allowed for faster approval during the height of the pandemic. This approval provides a level of confidence for those seeking a more traditionally developed vaccine. However, it's crucial to understand the nuances.
Limitations of the Novavax Approval: Why the Cautious Celebration?
While a significant milestone, the approval is not without limitations. The FDA's approval is specifically for individuals 18 years of age and older. This excludes adolescents and children, a key demographic in the fight against COVID-19. Further, the approval is only for a two-dose primary series. The data on booster shots using the Novavax vaccine is still under review.
This limited approval raises several questions:
- Market Share: Will the Novavax vaccine gain significant traction in a market already saturated with readily available mRNA vaccines? The convenience and wider age range of the mRNA vaccines might continue to make them the preferred choice for many.
- Booster Strategy: The lack of booster approval creates uncertainty for those who initially received the Novavax vaccine. What will their booster options be? Will they need to switch to an mRNA booster? This remains unclear.
- Global Impact: The FDA's approval could influence vaccination efforts globally. However, the limited age range and lack of booster data may limit its widespread adoption internationally.
How Does Novavax Differ from mRNA Vaccines?
Unlike Pfizer-BioNTech and Moderna's mRNA vaccines, Novavax utilizes a more traditional protein subunit technology. This technology uses harmless pieces of the virus's protein to trigger an immune response. Many individuals hesitant about mRNA vaccines may find this approach more acceptable. This difference in technology is a key selling point for Novavax.
What's Next for Novavax and the COVID-19 Vaccine Landscape?
The future of the Novavax vaccine hinges on several factors, including its uptake rate, the ongoing evaluation of booster shots, and the evolving COVID-19 landscape. The FDA’s approval, while a win for Novavax, is only a first step. Further research and data are needed to determine its long-term efficacy and role in the overall fight against the virus. The availability and ongoing clinical trials regarding booster shots will be key to its future success.
The Novavax approval represents a nuanced development in the COVID-19 vaccine landscape. While providing an alternative for some, its limitations highlight the ongoing complexities of vaccine development and distribution. Individuals considering the Novavax vaccine should consult their healthcare provider to determine if it's the right choice for them.
Keywords: Novavax, COVID-19 vaccine, FDA approval, protein subunit vaccine, mRNA vaccine, vaccine limitations, booster shot, vaccine safety, vaccine efficacy, COVID-19, pandemic, public health.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Approved By FDA, But With Limitations. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Final Toluca Vs America Confirmada La Fecha Y Hora De La Liga Mx 2025
May 20, 2025
Final Toluca Vs America Confirmada La Fecha Y Hora De La Liga Mx 2025
May 20, 2025 -
 Therapys Impact On The Brain Mechanisms Of Change And Neurological Outcomes
May 20, 2025
Therapys Impact On The Brain Mechanisms Of Change And Neurological Outcomes
May 20, 2025 -
 Go Jos Eurovision Exit And Australian Horror Films Legal Trouble
May 20, 2025
Go Jos Eurovision Exit And Australian Horror Films Legal Trouble
May 20, 2025 -
 The Scars Of Success Olympic Gold Medalists Account Of Abusive Coaching
May 20, 2025
The Scars Of Success Olympic Gold Medalists Account Of Abusive Coaching
May 20, 2025 -
 Eurovision 2023 What Went Wrong For Remember Monday
May 20, 2025
Eurovision 2023 What Went Wrong For Remember Monday
May 20, 2025
Latest Posts
-
 Billions Flow Into Bitcoin Etfs A Look At The Recent Investment Surge
May 20, 2025
Billions Flow Into Bitcoin Etfs A Look At The Recent Investment Surge
May 20, 2025 -
 Weaning Your Child Pacifiers And Thumb Sucking A Practical Approach
May 20, 2025
Weaning Your Child Pacifiers And Thumb Sucking A Practical Approach
May 20, 2025 -
 Three Decades On The Bench Reflecting On Alito And Roberts Supreme Court Impact
May 20, 2025
Three Decades On The Bench Reflecting On Alito And Roberts Supreme Court Impact
May 20, 2025 -
 Balis Plea International Cooperation For Safer More Respectful Tourism
May 20, 2025
Balis Plea International Cooperation For Safer More Respectful Tourism
May 20, 2025 -
 Alito And Roberts Supreme Court Legacy A Look At Their Near 30 Year Tenure
May 20, 2025
Alito And Roberts Supreme Court Legacy A Look At Their Near 30 Year Tenure
May 20, 2025
