Novavax COVID-19 Vaccine Gets FDA Approval: Understanding The Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Gets FDA Approval: Understanding the Usage Restrictions
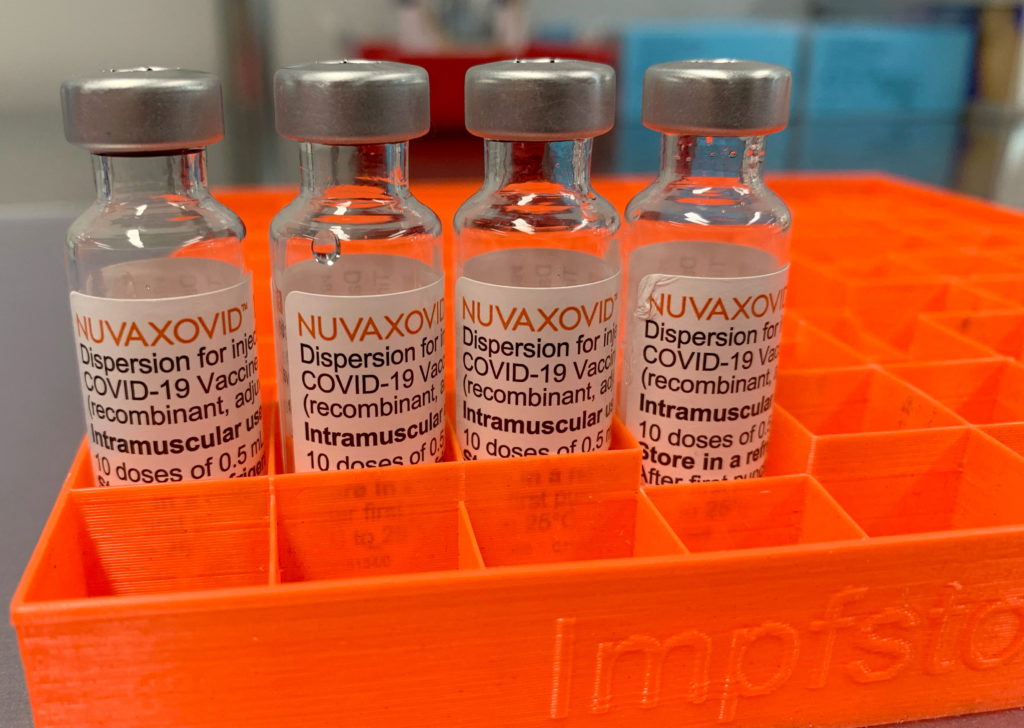
The Novavax COVID-19 vaccine, Nuvaxovid, has finally received full approval from the U.S. Food and Drug Administration (FDA). This marks a significant milestone for the vaccine, which has been available under Emergency Use Authorization (EUA) since July 2022. However, understanding the nuances of its approval, particularly the usage restrictions, is crucial for both healthcare providers and the public. This article will break down the key aspects of the FDA approval and clarify who can and cannot receive the Novavax vaccine.
What Makes the Novavax Vaccine Different?
Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, or the viral vector vaccine from Johnson & Johnson, Novavax uses a more traditional technology. It employs a protein subunit approach, using harmless pieces of the virus to trigger an immune response. This difference might appeal to individuals hesitant about mRNA technology, though efficacy remains comparable to other authorized vaccines. More information on vaccine technology comparisons can be found on the .
FDA Approval: A Closer Look
The FDA's full approval signifies a rigorous review of the vaccine's safety and efficacy data. This process goes beyond the EUA, providing a higher level of assurance regarding its long-term effects and suitability for widespread use. This approval covers individuals 18 years of age and older.
Understanding the Usage Restrictions
While the FDA approval is a positive development, it's essential to understand the existing usage restrictions:
-
Age Restriction: Currently, the Novavax vaccine is only fully approved for individuals 18 years of age and older. Children and adolescents require alternative vaccines authorized for their age group.
-
Prior Infection: While previous COVID-19 infection doesn't preclude receiving the Novavax vaccine, individuals who have recently recovered from COVID-19 may wish to consult their doctor to determine the optimal timing for vaccination.
-
Allergies: As with any vaccine, individuals with known allergies to any component of the Novavax vaccine should avoid receiving it. It's crucial to discuss any allergies with a healthcare professional before vaccination. Severe allergic reactions are rare but possible.
Who Should Consider the Novavax Vaccine?
The Novavax vaccine presents a viable option for several groups:
- Individuals hesitant about mRNA vaccines: The protein subunit technology may be a more appealing alternative for those concerned about mRNA vaccines.
- Adults seeking a fully approved vaccine: The full FDA approval provides reassurance for those seeking a rigorously tested vaccine.
Important Note: This article provides general information. Always consult your doctor or healthcare provider to determine if the Novavax vaccine is suitable for you based on your individual health circumstances and medical history.
What's Next for the Novavax Vaccine?
The FDA's full approval is likely to increase the vaccine's availability and accessibility. Further research and trials might expand its use to younger age groups in the future. The long-term effects of the vaccine continue to be monitored.
Disclaimer: This article is intended for informational purposes only and should not be considered medical advice. Always consult with a healthcare professional before making any decisions related to your health or treatment.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Approval: Understanding The Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 U S Treasury Yields Dip As Federal Reserve Hints At Single 2025 Rate Cut
May 21, 2025
U S Treasury Yields Dip As Federal Reserve Hints At Single 2025 Rate Cut
May 21, 2025 -
 Eu Uk Brexit Talks A Deal Or A Political Crisis
May 21, 2025
Eu Uk Brexit Talks A Deal Or A Political Crisis
May 21, 2025 -
 Tom Aspinall Contract Talks And Jon Jones Retirement Hint Whats Next For The Ufc
May 21, 2025
Tom Aspinall Contract Talks And Jon Jones Retirement Hint Whats Next For The Ufc
May 21, 2025 -
 Ufc News Jon Jones Hints At Retirement Amidst Aspinall Contract Delay
May 21, 2025
Ufc News Jon Jones Hints At Retirement Amidst Aspinall Contract Delay
May 21, 2025 -
 Jon Jones Explosive Claim Ufc Concealed Aspinalls Injury Status
May 21, 2025
Jon Jones Explosive Claim Ufc Concealed Aspinalls Injury Status
May 21, 2025
Latest Posts
-
 Prostate Cancer Diagnosis For President Joe Biden Implications And Response
May 21, 2025
Prostate Cancer Diagnosis For President Joe Biden Implications And Response
May 21, 2025 -
 Tom Aspinall Contract Talks And Jon Jones Retirement Hint Whats Next For The Ufc
May 21, 2025
Tom Aspinall Contract Talks And Jon Jones Retirement Hint Whats Next For The Ufc
May 21, 2025 -
 Fda Greenlights Novavax Covid 19 Vaccine But With Significant Restrictions
May 21, 2025
Fda Greenlights Novavax Covid 19 Vaccine But With Significant Restrictions
May 21, 2025 -
 Balis Tourism Overhaul Stricter Guidelines To Curb Negative Tourist Behavior
May 21, 2025
Balis Tourism Overhaul Stricter Guidelines To Curb Negative Tourist Behavior
May 21, 2025 -
 Self Driving Cars In The Uk Ubers 2027 Prediction And Industry Outlook
May 21, 2025
Self Driving Cars In The Uk Ubers 2027 Prediction And Industry Outlook
May 21, 2025
