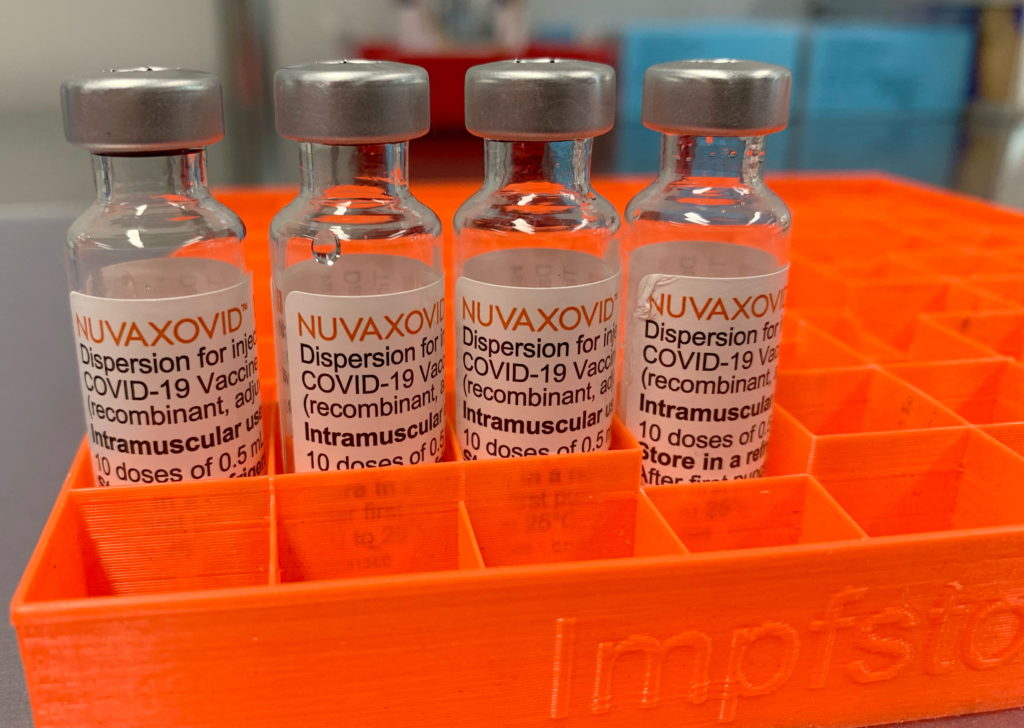
Restricted Use: FDA Authorizes Novavax COVID-19 Vaccine
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Restricted Use: FDA Authorizes Novavax COVID-19 Vaccine for Limited Populations
The FDA has granted Emergency Use Authorization (EUA) for the Novavax COVID-19 vaccine, but with significant limitations. This authorization, while a milestone for Novavax, marks a more cautious approach compared to other vaccines already on the market. Understanding the specifics of this restricted use is crucial for both healthcare providers and the public.
Why the Restricted Authorization?
The FDA's decision to grant only a restricted EUA is based on several factors. While the vaccine demonstrated efficacy and safety in clinical trials, its arrival relatively late in the pandemic means that its overall impact is likely to be smaller compared to vaccines already widely deployed. The authorization is further limited due to the availability of other, already-authorized vaccines with broader usage and demonstrated efficacy against currently circulating variants. This restricted authorization emphasizes a targeted approach, focusing on specific populations where the benefits might outweigh the potential risks, and where other vaccines might not be as suitable.
Who Can Receive the Novavax Vaccine Under the EUA?
The EUA specifically authorizes the Novavax COVID-19 vaccine (NVX-CoV2373) for individuals 18 years of age and older. However, the FDA stresses that it's particularly relevant for individuals who:
- Cannot or will not receive mRNA vaccines: This group includes individuals with specific medical conditions or strong personal preferences against mRNA technology. The Novavax vaccine utilizes a different protein-based technology, offering an alternative option.
- Have had adverse reactions to other COVID-19 vaccines: While rare, some individuals experience significant adverse reactions to mRNA vaccines. The Novavax vaccine provides a different avenue for vaccination in these cases.
Understanding the Novavax Vaccine Technology:
Unlike the Pfizer-BioNTech and Moderna vaccines which use mRNA technology, Novavax employs a protein subunit technology. This involves using harmless pieces of the virus (spike proteins) to trigger an immune response. This technology has been used in other vaccines for years, potentially making it a more familiar and accepted option for some individuals hesitant about newer technologies. For more information on the different COVID-19 vaccine technologies, you can consult the .
What are the potential benefits and risks?
The potential benefits include protection against severe COVID-19 illness, hospitalization, and death. However, like all vaccines, the Novavax vaccine carries potential risks, including mild to moderate side effects such as pain at the injection site, fatigue, headache, muscle aches, and nausea. Serious side effects are rare but possible. Individuals should consult their healthcare provider to discuss potential benefits and risks in relation to their individual health status.
Looking Ahead:
The FDA's restricted EUA for the Novavax vaccine presents a nuanced situation. While it offers a valuable alternative for certain populations, it's not a game-changer in the broader context of the ongoing pandemic. The long-term impact and utilization of the Novavax vaccine will depend on factors such as public acceptance, vaccine distribution, and the continuing evolution of the virus itself. Further research and monitoring of the vaccine's effectiveness against emerging variants will be crucial. Staying informed through reliable sources like the CDC and FDA websites is vital for making informed decisions about COVID-19 vaccination.
Call to Action: Consult your healthcare provider to discuss whether the Novavax vaccine is the right choice for you.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Restricted Use: FDA Authorizes Novavax COVID-19 Vaccine. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Sundance Hit Together Under Fire Copyright Suit Claims Blatant Rip Off By Alison Brie And Dave Franco
May 20, 2025
Sundance Hit Together Under Fire Copyright Suit Claims Blatant Rip Off By Alison Brie And Dave Franco
May 20, 2025 -
 Matthew Radaljs Account Of Life In A Chinese Prison
May 20, 2025
Matthew Radaljs Account Of Life In A Chinese Prison
May 20, 2025 -
 Copyright Lawsuit Hits Australian Body Horror Eurovision Loses Go Jo
May 20, 2025
Copyright Lawsuit Hits Australian Body Horror Eurovision Loses Go Jo
May 20, 2025 -
 Post Musk Era For Doge Analyzing The Impact Of Agency Overhauls And Budget Reductions
May 20, 2025
Post Musk Era For Doge Analyzing The Impact Of Agency Overhauls And Budget Reductions
May 20, 2025 -
 Moodys Downgrade Ignored Stock Market Climbs On S And P 500 Strength
May 20, 2025
Moodys Downgrade Ignored Stock Market Climbs On S And P 500 Strength
May 20, 2025
Latest Posts
-
 From Gold Medal To Broken Spirit An Olympians Struggle With Coaching Pressure
May 20, 2025
From Gold Medal To Broken Spirit An Olympians Struggle With Coaching Pressure
May 20, 2025 -
 Ufc Fans Erupt Over Jon Jones Latest Jibe At Tom Aspinall The Strip The Duck Controversy
May 20, 2025
Ufc Fans Erupt Over Jon Jones Latest Jibe At Tom Aspinall The Strip The Duck Controversy
May 20, 2025 -
 Femicide A Deep Dive Into The Causes Behind The Growing Number Of Incidents
May 20, 2025
Femicide A Deep Dive Into The Causes Behind The Growing Number Of Incidents
May 20, 2025 -
 Wall Street Defies Moody S S And P 500 Dow And Nasdaq Surge Higher
May 20, 2025
Wall Street Defies Moody S S And P 500 Dow And Nasdaq Surge Higher
May 20, 2025 -
 Urgent Peace Push Trump Announces Immediate Russia Ukraine Talks
May 20, 2025
Urgent Peace Push Trump Announces Immediate Russia Ukraine Talks
May 20, 2025
