Conditional FDA Approval: Novavax COVID-19 Vaccine And Its Restricted Use
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Conditional FDA Approval: Novavax COVID-19 Vaccine and Its Restricted Use
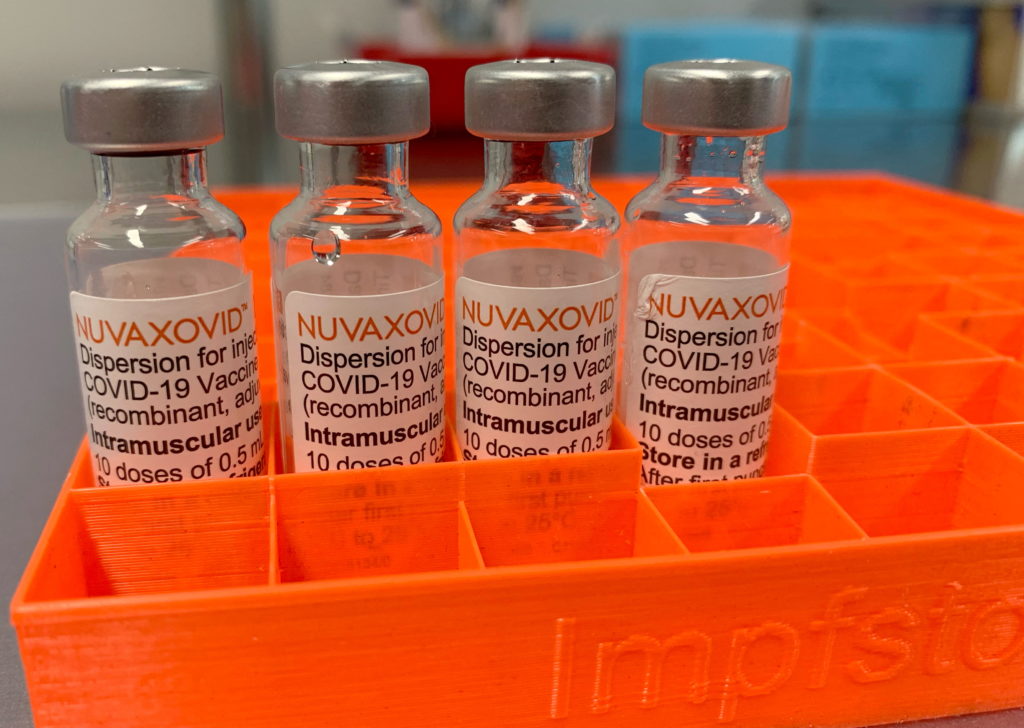
The Novavax COVID-19 vaccine, Nuvaxovid, finally received conditional approval from the FDA in July 2023, marking a significant development in the fight against the pandemic. However, this approval comes with crucial caveats, specifically regarding its restricted use and limited market impact compared to already established vaccines. Understanding these limitations is key to accurately assessing its role in the ongoing vaccination efforts.
A Latecomer to the Market:
Unlike Pfizer-BioNTech and Moderna's mRNA vaccines, which dominated the early stages of the vaccination rollout, Novavax's protein-subunit vaccine utilizes a more traditional approach. This technology has been around for decades and is known for its generally well-tolerated safety profile. However, this familiar technology also contributed to the vaccine's delayed arrival, facing production challenges and slower regulatory processes. This delay means Nuvaxovid is entering a market already saturated with other vaccine options, significantly impacting its potential reach.
The FDA's Conditional Approval and Restrictions:
The FDA's conditional approval wasn't a blanket endorsement. It's crucial to understand that the approval is conditional, meaning ongoing monitoring and further data collection are required to confirm the vaccine's long-term safety and efficacy. Furthermore, the approval is specifically for individuals 18 years of age and older. This differs from some competitor vaccines authorized for younger age groups. The restricted use also stems from the limited real-world data available compared to the more established vaccines.
Why the Restricted Use?
Several factors contribute to the restricted use of the Novavax vaccine:
- Limited Clinical Trial Data: While clinical trials demonstrated efficacy, the sample size and duration were comparatively smaller than those of the leading vaccines, leading to some uncertainty regarding long-term effects.
- Market Saturation: The availability of other highly effective vaccines diminishes the immediate need for a new option, especially one with a more limited track record.
- Production and Distribution Challenges: Scaling up production and ensuring smooth distribution proved to be a hurdle for Novavax, further limiting its availability.
Nuvaxovid's Potential Role:
Despite its late entry and restricted use, the Novavax vaccine could still play a vital role. Its protein-subunit technology might appeal to individuals hesitant about mRNA vaccines, offering an alternative option based on a more familiar vaccine platform. This could potentially increase vaccination rates among those who were previously reluctant. Further research and data collection will be crucial in determining its long-term efficacy and overall impact on public health.
Looking Ahead:
The Novavax vaccine's conditional approval highlights the ongoing evolution of the COVID-19 vaccine landscape. While it's a latecomer facing considerable hurdles, its potential to reach vaccine-hesitant populations and offer a technologically distinct option remains. Continued monitoring of its safety and efficacy, along with further clinical trials, will be essential to fully assess its long-term contribution to the global fight against COVID-19. For the most up-to-date information on COVID-19 vaccines, consult the and your healthcare provider.
Keywords: Novavax, COVID-19 vaccine, Nuvaxovid, FDA approval, conditional approval, protein-subunit vaccine, mRNA vaccine, vaccine hesitancy, vaccination rates, public health, COVID-19 pandemic, clinical trials, vaccine safety, vaccine efficacy.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval: Novavax COVID-19 Vaccine And Its Restricted Use. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Jamie Lee Curtis On Lindsay Lohan Honesty And A Lasting Bond
May 20, 2025
Jamie Lee Curtis On Lindsay Lohan Honesty And A Lasting Bond
May 20, 2025 -
 Lineker Exit Match Of The Day Future Uncertain
May 20, 2025
Lineker Exit Match Of The Day Future Uncertain
May 20, 2025 -
 Exclusive Interview Jamie Lee Curtis On Her Relationship With Lindsay Lohan After Freaky Friday
May 20, 2025
Exclusive Interview Jamie Lee Curtis On Her Relationship With Lindsay Lohan After Freaky Friday
May 20, 2025 -
 Peaky Blinders Creator Reveals New Series Details And Significant Shift
May 20, 2025
Peaky Blinders Creator Reveals New Series Details And Significant Shift
May 20, 2025 -
 Mma News Fan Fury Over Jon Jones Latest Comments On Tom Aspinall
May 20, 2025
Mma News Fan Fury Over Jon Jones Latest Comments On Tom Aspinall
May 20, 2025
Latest Posts
-
 Urgent Security Alert Legal Aid System Hacked Exposing Criminal And Private Client Information
May 21, 2025
Urgent Security Alert Legal Aid System Hacked Exposing Criminal And Private Client Information
May 21, 2025 -
 Jon Jones Explosive Claim Ufc Concealed Aspinalls Injury Status
May 21, 2025
Jon Jones Explosive Claim Ufc Concealed Aspinalls Injury Status
May 21, 2025 -
 Buy Now Pay Later Key Changes In The New Consumer Protection Rules
May 21, 2025
Buy Now Pay Later Key Changes In The New Consumer Protection Rules
May 21, 2025 -
 Moodys Downgrade Unfazed Stock Market Soars S And P 500 Hits Six Day High
May 21, 2025
Moodys Downgrade Unfazed Stock Market Soars S And P 500 Hits Six Day High
May 21, 2025 -
 Ufc News Jon Jones Future Hangs In The Balance After Cryptic Tweet Aspinall Talks Breakdown
May 21, 2025
Ufc News Jon Jones Future Hangs In The Balance After Cryptic Tweet Aspinall Talks Breakdown
May 21, 2025
