FDA Approval For Novavax COVID-19 Vaccine: Use Restrictions Explained
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Approval for Novavax COVID-19 Vaccine: Use Restrictions Explained
The FDA's full approval of the Novavax COVID-19 vaccine marks a significant milestone in the fight against the pandemic. However, understanding the nuances of its approval, including the specific use restrictions, is crucial for both healthcare providers and the public. This article clarifies the FDA's decision and details the limitations surrounding the vaccine's use.
A Closer Look at the Novavax Approval:
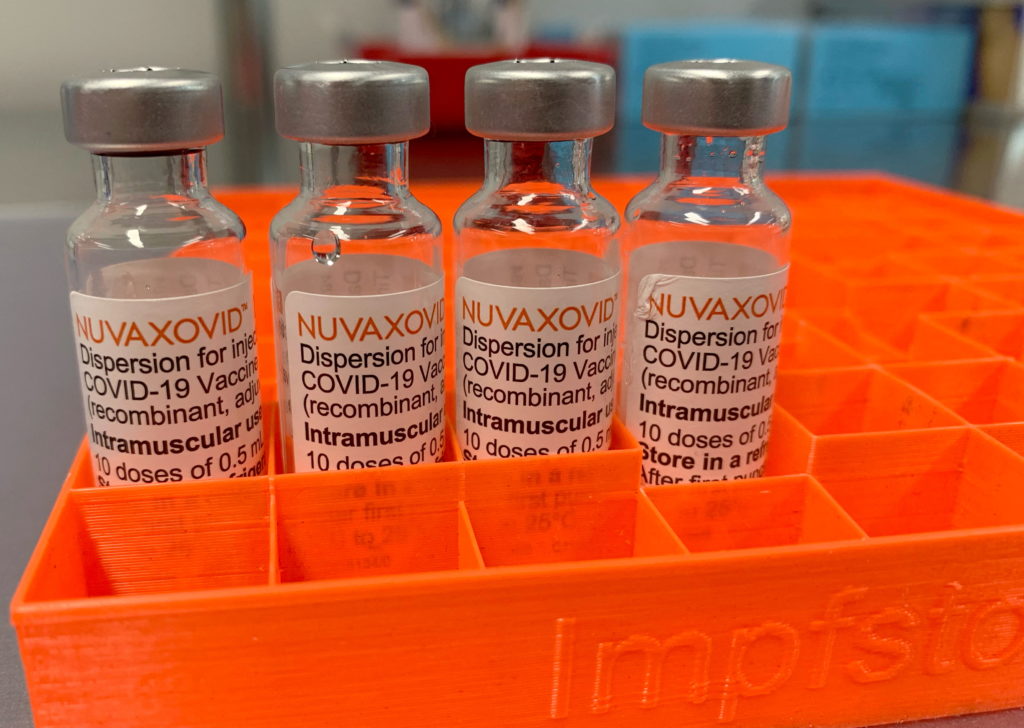
The Novavax vaccine, marketed under the brand name Nuvaxovid, uses a different technology compared to mRNA vaccines like Pfizer-BioNTech and Moderna. It's a protein subunit vaccine, utilizing a lab-made version of the spike protein found on the surface of the SARS-CoV-2 virus. This protein triggers an immune response, helping the body fight off infection. The FDA's approval signifies that the vaccine has met rigorous safety and effectiveness standards. This approval is distinct from the previous Emergency Use Authorization (EUA), offering a greater degree of regulatory certainty.
Understanding the Use Restrictions:
While the full approval is positive news, it's important to note that the FDA's approval is not without limitations. The vaccine is currently authorized for:
- Individuals 18 years of age and older: This age restriction is a key factor. Clinical trials did not include younger age groups, hence the limitation. Further research and trials are needed to assess its safety and efficacy in adolescents and children.
- Two-dose primary series: The approved regimen consists of two doses administered three weeks apart. This is consistent with the original clinical trial data. Information regarding booster doses is still evolving.
- Specific indications: The approval focuses on preventing COVID-19 disease caused by the original SARS-CoV-2 virus. The effectiveness against emerging variants is an ongoing area of research and may influence future recommendations.
What the Restrictions Mean for You:
These restrictions mean that the Novavax vaccine is not a one-size-fits-all solution. Individuals under 18 should not receive this vaccine at this time. Furthermore, those seeking protection against specific variants might need to consider other vaccination strategies alongside ongoing monitoring of variant-specific efficacy data. Consult your physician to discuss which COVID-19 vaccine is most suitable for your individual needs and circumstances.
Addressing Common Concerns:
Many are curious about the Novavax vaccine's safety profile, particularly in comparison to mRNA vaccines. The FDA's approval process rigorously evaluated safety data, concluding the benefits outweigh the risks for the approved population. However, as with all vaccines, potential side effects exist, including pain at the injection site, fatigue, headache, muscle aches, and joint pain. These side effects are usually mild and temporary. Serious side effects are rare.
Looking Ahead: Future Developments and Research:
Ongoing research continues to assess the Novavax vaccine's long-term efficacy and safety, as well as its effectiveness against emerging variants. Further studies are crucial to determine its potential use in younger populations and its role in future booster campaigns. The FDA will continue to monitor its safety and effectiveness post-market.
Conclusion:
The FDA's full approval of the Novavax COVID-19 vaccine is a welcome development, offering an alternative vaccine option for eligible adults. Understanding the specific use restrictions is paramount for ensuring safe and effective vaccination strategies. Always consult with your healthcare provider to make informed decisions regarding COVID-19 vaccination. Stay informed about updates and ongoing research through reputable sources like the CDC and FDA websites.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine: Use Restrictions Explained. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Femicide Statistics And Trends A Growing Threat To Women Worldwide
May 20, 2025
Femicide Statistics And Trends A Growing Threat To Women Worldwide
May 20, 2025 -
 Air Force Ones Transformation Whats Changing In The Presidential Jet
May 20, 2025
Air Force Ones Transformation Whats Changing In The Presidential Jet
May 20, 2025 -
 Is Jon Jones Leaving The Ufc Aspinall Stalemate And Retirement Hints Spark Debate
May 20, 2025
Is Jon Jones Leaving The Ufc Aspinall Stalemate And Retirement Hints Spark Debate
May 20, 2025 -
 Putins Calculated Snub Demonstrating Independence From Trump
May 20, 2025
Putins Calculated Snub Demonstrating Independence From Trump
May 20, 2025 -
 Arthur Claims Victory Intense Aussie Coaching Clash In Super League
May 20, 2025
Arthur Claims Victory Intense Aussie Coaching Clash In Super League
May 20, 2025
Latest Posts
-
 Urgent Security Alert Legal Aid System Hacked Exposing Criminal And Private Client Information
May 21, 2025
Urgent Security Alert Legal Aid System Hacked Exposing Criminal And Private Client Information
May 21, 2025 -
 Jon Jones Explosive Claim Ufc Concealed Aspinalls Injury Status
May 21, 2025
Jon Jones Explosive Claim Ufc Concealed Aspinalls Injury Status
May 21, 2025 -
 Buy Now Pay Later Key Changes In The New Consumer Protection Rules
May 21, 2025
Buy Now Pay Later Key Changes In The New Consumer Protection Rules
May 21, 2025 -
 Moodys Downgrade Unfazed Stock Market Soars S And P 500 Hits Six Day High
May 21, 2025
Moodys Downgrade Unfazed Stock Market Soars S And P 500 Hits Six Day High
May 21, 2025 -
 Ufc News Jon Jones Future Hangs In The Balance After Cryptic Tweet Aspinall Talks Breakdown
May 21, 2025
Ufc News Jon Jones Future Hangs In The Balance After Cryptic Tweet Aspinall Talks Breakdown
May 21, 2025
