FDA Approves Novavax COVID-19 Vaccine: Use Restrictions Explained
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Approves Novavax COVID-19 Vaccine: Use Restrictions Explained
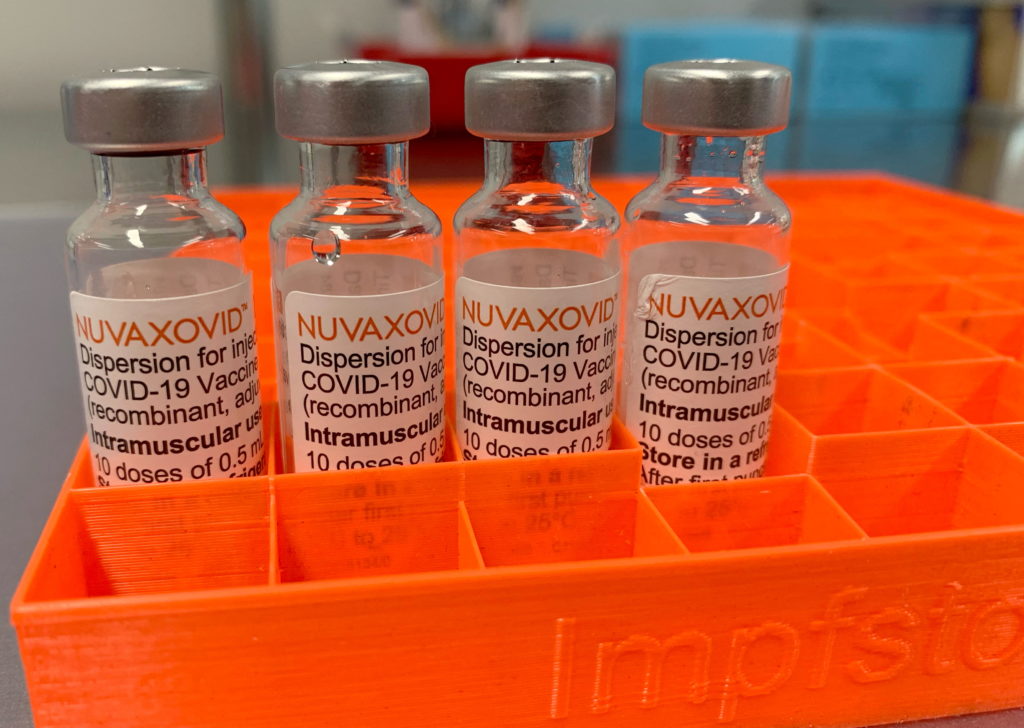
The FDA's approval of the Novavax COVID-19 vaccine marks a significant milestone in the fight against the pandemic. This protein-subunit vaccine offers a different approach than the mRNA vaccines already available, potentially appealing to individuals hesitant about the mRNA technology. However, understanding the nuances of its use and the associated restrictions is crucial. This article breaks down the key details of the FDA's approval and explains the limitations surrounding its use.
Understanding the Novavax Vaccine:
Unlike Pfizer-BioNTech and Moderna vaccines which utilize mRNA technology, Novavax's Nuvaxovid employs a more traditional protein-subunit approach. This means it uses lab-grown pieces of the virus's spike protein to trigger an immune response. Many see this as a more familiar and potentially less concerning technology compared to mRNA vaccines. This difference is a key factor in the vaccine's appeal to a segment of the population who have been hesitant to get vaccinated. The vaccine's mechanism of action involves stimulating the body to produce antibodies against the spike protein, preparing the immune system to fight off a COVID-19 infection.
FDA Approval and Authorization:
The FDA granted Emergency Use Authorization (EUA) for Nuvaxovid initially, and subsequently granted full licensure. This full approval signifies a rigorous review process, bolstering confidence in the vaccine's safety and efficacy. The approval covers individuals 18 years of age and older.
Use Restrictions and Considerations:
While the approval is a positive step, certain restrictions and considerations are important to understand:
-
Age Restrictions: Currently, the FDA's full approval is for adults 18 years and older. Further studies are needed to determine its safety and efficacy in younger age groups. This differs from some other COVID-19 vaccines authorized for use in younger populations.
-
Allergies: Individuals with a history of severe allergic reactions to any component of the vaccine should not receive it. As with all vaccines, close monitoring for allergic reactions is crucial immediately following vaccination. Consult your physician if you have any concerns regarding allergies.
-
Efficacy Data: While the clinical trials demonstrated a high level of efficacy against COVID-19, it's essential to understand that vaccine effectiveness can vary based on individual factors and evolving viral variants. The vaccine's effectiveness against newer variants is constantly being monitored and evaluated.
-
Two-Dose Regimen: Nuvaxovid is administered as a two-dose primary series, given three to eight weeks apart. Like other COVID-19 vaccines, booster shots might be recommended based on future guidance from public health authorities.
-
Storage and Handling: The vaccine requires specific storage and handling procedures to maintain its efficacy. This is crucial for ensuring its potency and effectiveness upon administration.
Addressing Vaccine Hesitancy:
The Novavax vaccine's approval provides an alternative for those hesitant about mRNA vaccines. This could contribute significantly to increasing vaccination rates globally. Open communication about the science behind the vaccine, along with addressing specific concerns, is key to overcoming vaccine hesitancy.
Conclusion:
The FDA approval of the Novavax COVID-19 vaccine is a welcome development, offering an additional tool in the fight against the pandemic. However, understanding the specific use restrictions and considerations is critical for both healthcare providers and individuals considering vaccination. Consult with your doctor to determine if the Novavax vaccine is the right choice for you. For more information, you can visit the and the . Stay informed and stay safe.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approves Novavax COVID-19 Vaccine: Use Restrictions Explained. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Lufthansa Plane Flies Unaided For 10 Minutes Following Co Pilots Medical Emergency
May 20, 2025
Lufthansa Plane Flies Unaided For 10 Minutes Following Co Pilots Medical Emergency
May 20, 2025 -
 Beyond The Action Exploring The Emotional Depth Of The Last Of Us
May 20, 2025
Beyond The Action Exploring The Emotional Depth Of The Last Of Us
May 20, 2025 -
 Expert Mlb Betting Picks Based On Splits May 19th
May 20, 2025
Expert Mlb Betting Picks Based On Splits May 19th
May 20, 2025 -
 North Texas Weather Update Storms Clear Cold Front Incoming Tuesday
May 20, 2025
North Texas Weather Update Storms Clear Cold Front Incoming Tuesday
May 20, 2025 -
 Sean Combs Faces Serious Charges Cassie Venturas Testimony Holds The Key
May 20, 2025
Sean Combs Faces Serious Charges Cassie Venturas Testimony Holds The Key
May 20, 2025
Latest Posts
-
 Urgent Security Alert Legal Aid System Hacked Exposing Criminal And Private Client Information
May 21, 2025
Urgent Security Alert Legal Aid System Hacked Exposing Criminal And Private Client Information
May 21, 2025 -
 Jon Jones Explosive Claim Ufc Concealed Aspinalls Injury Status
May 21, 2025
Jon Jones Explosive Claim Ufc Concealed Aspinalls Injury Status
May 21, 2025 -
 Buy Now Pay Later Key Changes In The New Consumer Protection Rules
May 21, 2025
Buy Now Pay Later Key Changes In The New Consumer Protection Rules
May 21, 2025 -
 Moodys Downgrade Unfazed Stock Market Soars S And P 500 Hits Six Day High
May 21, 2025
Moodys Downgrade Unfazed Stock Market Soars S And P 500 Hits Six Day High
May 21, 2025 -
 Ufc News Jon Jones Future Hangs In The Balance After Cryptic Tweet Aspinall Talks Breakdown
May 21, 2025
Ufc News Jon Jones Future Hangs In The Balance After Cryptic Tweet Aspinall Talks Breakdown
May 21, 2025
