Restricted Use: FDA Grants Approval To Novavax COVID-19 Vaccine
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Restricted Use: FDA Grants Approval to Novavax COVID-19 Vaccine
The FDA's conditional approval of the Novavax COVID-19 vaccine marks a significant development in the fight against the pandemic, but with limitations.
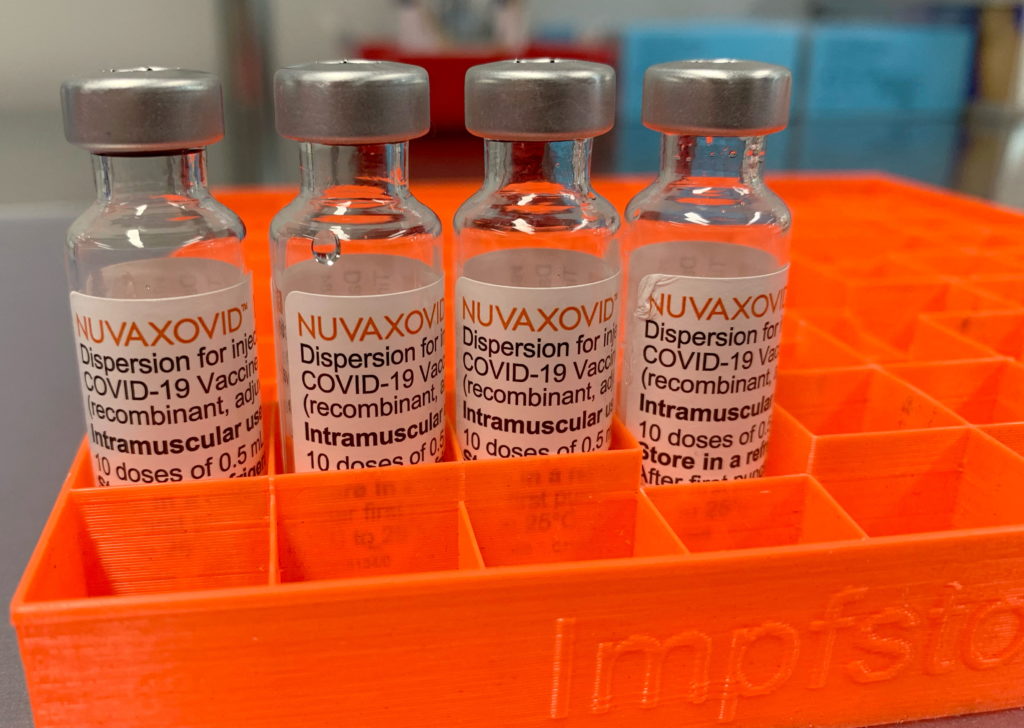
The news broke late last week: the US Food and Drug Administration (FDA) has granted Emergency Use Authorization (EUA) to the Novavax COVID-19 vaccine, Nuvaxovid. This protein-subunit vaccine, a different technology than the mRNA vaccines from Pfizer-BioNTech and Moderna, offers a potential alternative for those hesitant about mRNA technology or seeking a different option. However, the approval comes with a crucial caveat: restricted use. This means the vaccine isn't being widely rolled out immediately. Let's delve into the details.
Why the Restricted Use Designation?
The FDA's decision to grant EUA with restrictions is not unusual for new vaccines. It allows for the vaccine to be used while further safety and efficacy data is collected. In the case of Nuvaxovid, the FDA cited the need for more data on long-term effects and certain population groups. This cautious approach prioritizes public health and safety.
Several factors likely contributed to the restricted use:
- Smaller-Scale Clinical Trials: While the trials demonstrated efficacy, they involved fewer participants compared to the mRNA vaccine trials, leading to a need for more data to confirm long-term safety and efficacy.
- Manufacturing Challenges: The production of protein-subunit vaccines can sometimes be more complex and slower than mRNA vaccines, impacting initial availability.
- Evolving Pandemic Landscape: The COVID-19 pandemic continues to evolve, with new variants emerging. The FDA wants to ensure the vaccine's effectiveness against these variants before widespread distribution.
What Does "Restricted Use" Mean in Practice?
The restricted use designation means that the vaccine's rollout will be more targeted initially. The FDA will likely monitor its use closely, focusing on specific demographics or situations where the benefits outweigh the potential risks. This phased approach allows for better data collection and safety monitoring as the vaccine is deployed. This is a standard practice and shouldn't be misinterpreted as a sign of ineffectiveness or significant safety concerns.
Novavax Vaccine: A Different Approach
Nuvaxovid represents a different technological approach to COVID-19 vaccination. Unlike mRNA vaccines, which use messenger RNA to instruct cells to produce a viral protein, Nuvaxovid uses a lab-made protein to trigger an immune response. This protein-subunit technology has been used for other vaccines for years, potentially appealing to individuals concerned about the newer mRNA technology.
Looking Ahead: What's Next for Nuvaxovid?
The FDA's decision is a positive step, offering another tool in the fight against COVID-19. The restricted use authorization allows for continued monitoring and data collection, paving the way for a potential expansion of its use in the future. The Centers for Disease Control and Prevention (CDC) will play a vital role in guiding the strategic distribution and implementation of the vaccine. It's crucial to stay updated on official announcements from the FDA and CDC regarding the vaccine's availability and recommended use.
Further Reading:
- [Link to FDA website regarding the approval]
- [Link to CDC website on COVID-19 vaccines]
Disclaimer: This article provides information for educational purposes only and should not be considered medical advice. Consult with a healthcare professional for any health concerns or before making any decisions related to your health or treatment.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Restricted Use: FDA Grants Approval To Novavax COVID-19 Vaccine. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Family Tragedy Train Collision Leaves Two Adults Dead Children Injured And Missing
May 21, 2025
Family Tragedy Train Collision Leaves Two Adults Dead Children Injured And Missing
May 21, 2025 -
 Us Treasury Yields Fall As Fed Hints At One 2025 Rate Decrease
May 21, 2025
Us Treasury Yields Fall As Fed Hints At One 2025 Rate Decrease
May 21, 2025 -
 Peaky Blinders Creator Confirms New Series A Major Change Awaits
May 21, 2025
Peaky Blinders Creator Confirms New Series A Major Change Awaits
May 21, 2025 -
 Tense Brexit Talks Eu Accuses Uk Of Betrayal Deadlock Looms
May 21, 2025
Tense Brexit Talks Eu Accuses Uk Of Betrayal Deadlock Looms
May 21, 2025 -
 Jon Jones Cryptic I M Done Message Ufc Career Hanging In The Balance After Aspinall Stalemate
May 21, 2025
Jon Jones Cryptic I M Done Message Ufc Career Hanging In The Balance After Aspinall Stalemate
May 21, 2025
Latest Posts
-
 10 Minutes Of Unpiloted Flight Lufthansa Incident Report Highlights Safety Concerns
May 21, 2025
10 Minutes Of Unpiloted Flight Lufthansa Incident Report Highlights Safety Concerns
May 21, 2025 -
 Market Rally Continues S And P 500s Six Day Winning Streak And Positive Market Sentiment
May 21, 2025
Market Rally Continues S And P 500s Six Day Winning Streak And Positive Market Sentiment
May 21, 2025 -
 St Louis Tornado Community Rallies To Rebuild After Devastating Storm
May 21, 2025
St Louis Tornado Community Rallies To Rebuild After Devastating Storm
May 21, 2025 -
 Jon Jones Vs Tom Aspinall Analyzing The Controversy Behind Jones Latest Statement
May 21, 2025
Jon Jones Vs Tom Aspinall Analyzing The Controversy Behind Jones Latest Statement
May 21, 2025 -
 Weaning Your Child Off Pacifiers And Thumb Sucking Age Methods And Challenges
May 21, 2025
Weaning Your Child Off Pacifiers And Thumb Sucking Age Methods And Challenges
May 21, 2025
